Abstract
Aims
To examine the effects of high ambient temperature (‘heat stressor’) on parasympathetically mediated cardiovascular reflexes (power of respiratory sinus dysrhythmia; change in heart rate elicited by change in posture from lying to standing [‘30 : 15 ratio’]).
Methods
Twelve healthy male volunteers participated in four weekly sessions, each of which was associated with one treatment condition (placebo at an ambient temperature of 22 °C; propranolol 40 mg at 22 °C; placebo at 40 °C; propranolol 40 mg at 40 °C), according to a balanced double-blind design. Heart rate was recorded by ECG, finger tremor (7–12 Hz) with an accelerometer strapped to the middle finger of the nondominant hand, and sublingual temperature by a mercury thermometer. Power of finger tremor and the variations of the R-R intervals of the ECG were obtained from Fourier transformations of the data. Data were analysed by analysis of variance, with repeated measures using a significance criterion of P < 0.05; individual comparisons of active treatment with placebo and of data obtained at 40 °C with those obtained at 22 °C were made with Fisher's Least Significant Difference test.
Results
Heart rate was increased by the heat stressor, and this increase was abolished by propranolol. The heat stressor reduced the power of respiratory sinus dysrhythmia and the 30 : 15 ratio, and increased the power of physiological finger tremor. Propranolol did not affect heat stressor-induced changes in the parasympathetic cardiac reflexes, but reduced the heat stressor-induced enhancement of finger tremor.
Conclusions
The increase in the power of physiological finger tremor at high ambient temperature is consistent with sympathetic activation, whereas the reduction in the power of respiratory sinus dysrhythmia and 30 : 15 ratio indicates a decrease in parasympathetic activity. These results demonstrate that high ambient temperature may induce vagal withdrawal in the heart.
Keywords: cardiovascular reflexes, heart rate, heat stressor, physiological finger tremor, propranolol
Introduction
There is evidence that high ambient temperature (‘heat stressor’) increases impulse traffic in sympathetic fibres [1]. The enhancement of sympathetic activity by high ambient temperature is also reflected in increases in a number of sympathetically mediated functions, such as heart rate [2], power of physiological finger tremor [2], sensitivity of eccrine sweat glands to interdermally injected carbachol [3], rate of redilatation of the pupil after the cessation of a light stimulus [4]. Although it has been shown that some parasympathetically mediated functions (e.g. amplitude of the pupillary light reflex response [4], salivary output [2]) remain unaffected by the heat stressor, little is known about the effects of this variable on parasympathetically mediated cardiac functions. Therefore, we have examined the effects of high ambient temperature on two parasympathetically mediated cardiac reflexes: power of respiratory dysrhythmia [5], and the change in heart rate elicited by a change in posture from lying to standing (‘30 : 15 ratio’) [6]. Since the cardiac reflexes are heart-rate dependent [7], we used propranolol, a β-adrenoceptor antagonist with a demonstrated ability to reduce heart rate [8] to remove the increase in heart rate evoked by the heat stressor.
Some of the result presented in this paper have been communicated to the British Pharmacological Society [9].
Methods
Subjects
Twelve healthy drug-free male volunteers, aged 19–30 years, participated. Each subject completed a brief medical history and underwent a complete physical examination before inclusion in the study. All subjects reported compliance with the request to abstain from caffeine-containing beverages on each test day. All volunteers gave their written informed consent following a verbal explanation of the study and after reading a detailed information sheet. The study protocol was approved by the University of Nottingham Medical School Ethics Committee.
Drugs
Propranolol hydrochloride (40 mg) and placebo were prepared in identical capsules and administered orally.
Design
There were two environmental conditions (ambient temperature of 22 °C and 40 °C) and two drug conditions (propranolol hydrochloride 40 mg and placebo). Four treatment conditions were obtained by combining the two drug and environmental conditions: placebo/22 °C, propranolol/22 °C, placebo/40 °C, propranolol/40 °C. Each subject participated in four experimental sessions at weekly intervals, each session being associated with one of the treatment conditions. Subjects were allocated to treatment conditions and sessions double-blind, according to a balanced cross-over design.
Tests
Heart rate was continuously monitored using a 5-lead electrocardiograph (ECG: Life-Trace 18, Albany Instruments); interbeat (RR) intervals were calculated and the power of variation in RR intervals during 2.25 min ECG samples was computed using a BBC Master microcomputer equipped with a fast Fourier Transform ROM. The heart rate response to standing was measured as the ‘30 : 15 ratio’ (ratio of the longest RR interval, approximately 30 beats after standing, to the shortest RR interval, approximately 15 beats after standing). The respiratory component of the power of RR interval variation was measured during spontaneous breathing in the erect posture. Blood pressure was measured using an electroaneroid sphygmomanometer (Brethren Corporation), both in the supine and erect (after 2 min standing) positions. Oral temperature was recorded with a mercury thermometer. Finger tremor was recorded with an accelerometer taped to the terminal phalanx of the middle finger of the outstretched nondominant hand, as described by Arya et al. [2]. The amplified signal was analysed using a BBC Master microcomputer equipped with a Fast Fourier Transform ROM, which enabled computation of tremor power within each 1 Hz frequency band between 2 and 36 Hz. ‘Physiological tremor’ was defined as power within the 7–12 Hz frequency band [2].
Procedure
Upon arrival in the laboratory, each subject rested for 30 min. Then the oral temperature was measured, followed by the ingestion of the capsule. For the remainder of the session, the subject either stayed in the main laboratory (22 °C), or was transferred to the temperature-controlled hot-room (40 °C) 90 min after the ingestion of the capsule. Post-drug testing was carried out between 120 and 150 min after the ingestion of the capsule.
Data analysis
Group mean (± s.e. mean) data were calculated for each treatment condition. Two-factor (drug × temperature condition) analysis of variance, with repeated measures on both factors, was used. Individual comparisons between propranolol 40 mg and placebo, and between 40 °C and 22 °C, were made using Fisher's Least Significant Difference test with an a priori criterion of P < 0.05 (d.f = 11; criterion t = 1.8). In addition, the differences (mean, 95% CI) between propranolol and placebo, and 40 °C and 22 °C, were calculated.
Results
Oral temperature
The changes (mean ± s.e.mean) in oral temperature (°C) from pretreatment were as follows: placebo/22 °C: −0.38 ± 0.21; propranolol/22 °C: −0.50 ± 0.14; placebo/40 °C: 0.27 ± 0.07; propranolol/40 °C: 0.50 ± 0.11. There was a significant main effect of temperature condition (F1,11 = 48.6; P < 0.001); the main effect of drug treatment and the interaction were not statistically significant (F < 1 in both cases).
Heart rate
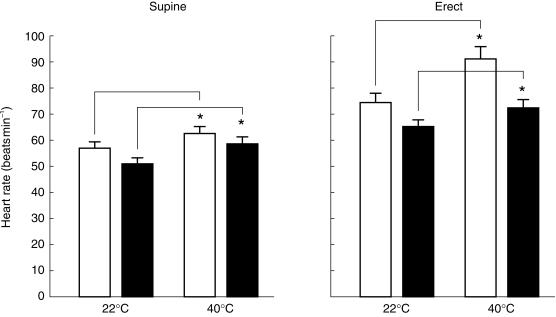
The effect of the treatment conditions on heart rate, measured in both the supine and the erect positions, are shown in Figure 1 and Table 1. The data obtained in the supine and erect positions were analysed separately. In the supine position, two-way anova revealed significant main effects of temperature condition (F1,11 = 27.8; P < 0.001) and drug treatment (F1,11 = 34.1; P < 0.001), but no significant temperature condition × drug treatment interaction (F1,11 = 2.1; P > 0.1). Compared with the 22 °C condition, the 40 °C condition significantly increased heart rate both in the presence of placebo (t = 3.1), and in the presence of propranolol (t = 4.4) (see Table 1). In the erect position, two-way anova revealed significant main effects of temperature condition (F1,11 = 26.4; P < 0.001) and drug treatment (F1,11 = 14.3; P < 0.01), but no significant temperature condition × drug treatment interaction (F < 1). Compared with the 22 °C condition, the 40 °C condition significantly increased heart rate both in the presence of placebo (t = 5.2), and in the presence of propranolol (t = 2.3) (see Table 1). Propranolol effectively counteracted the increase in heart rate evoked by the 40 °C condition, as evidenced by comparing the difference in heart rate between the placebo/22 °C and placebo/40 °C conditions with the difference in heart rate between the placebo/22 °C and propranolol/40 °C conditions. In the supine position, the difference (mean, 95% CI) between the placebo/22 °C and placebo/40 °C conditions was + 6.3 (+ 1.9, + 10.6), whereas the difference between the placebo/22 °C and propranolol/40 °C conditions was −1.3 (−5.0, + 2.3); in the erect position, the differences were + 16.7 (+ 5.6, + 27.7) and + 2.2 (−3.5, + 7.9), respectively.
Figure 1.
Heart rate measured in the supine (left) and erect (right) positions at the two temperature conditions (22 °C and 40 °C). Open columns: placebo; closed columns: propranolol 40 mg. The height of each column corresponds to the mean (n = 12), vertical bars are s.e. mean. Statistically significant differences between the two temperature conditions *P < 0.05.
Table 1.
Effect of high ambient temperature (difference from 22 °C condition; mean, 95% CI).
| Placebo | Propranolol (40 mg) | |
|---|---|---|
| Heart rate (beats min−1) | ||
| Supine | + 6.3 (+ 2.0, + 10.6) | + 7.7 (+ 3.2, + 12.2) |
| Erect | + 16.7 (+ 5.6, + 27.7) | + 7.4 (+ 1.8, + 13.0) |
| Sinus dysrhythmia (power; ms2) | −243.2 (−455.4, −31.2) | −430.8 (−863.2, + 1.2) |
| 30 : 15 ratio | −0.09 (−0.20, + 0.01) | −0.15 (−0.31, 0.00) |
| Finger tremor (power; arbitrary units) | + 25736 (+ 6766, + 44705) | + 8141 (−821, + 17105) |
Heart rate variability
The effects of the treatments on the power of sinus dysrhythmia in the erect position and the 30 : 15 ratio are shown in Figure 2 and Table 1. The absolute values are shown in Figure 2. anova of the sinus dysrhythmia data revealed a significant main effect of the temperature condition (F1,11 = 7.0; P < 0.05); there was no significant main effect of drug treatment (F1,11 = 1.4; P > 0.1), and no significant interaction (F < 1). Compared with the 22 °C condition, the 40 °C condition produced a significant decrease in power, both in the presence of placebo (t = 1.9) and in the presence of propranolol (t = 3.4) (see Table 1). anova of the 30 : 15 ratio data revealed a significant main effect of the temperature condition (F1,11 = 6.9; P < 0.05); there was no significant main effect of drug treatment and no significant interaction (F < 1 in both cases). Compared with the 22 °C condition, the 40 °C condition produced a significant decrease in power, both in the presence of placebo (t = 2.2) and in the presence of propranolol (t = 3.7) (see Table 1).
Figure 2.
Heart rate variability: power of sinus dysrhythmia (left) and 30 : 15 ratio (right). Open columns: placebo; closed columns: propranolol 40 mg. The height of each column corresponds to the mean (n = 12), vertical bars are s.e. mean. Statistically significant differences between the two temperature conditions *P < 0.05.
Blood pressure
The effects of the treatments on systolic and diastolic blood pressure, measured both in the supine and erect positions, are shown in Table 2. The data obtained for the four sets of blood pressure data (systolic/supine, systolic/erect, diastolic/supine, diastolic/erect) were analysed separately. Two-way anova revealed no significant main or interaction effects (P > 0.05, for all four sets of blood pressure data).
Table 2.
Blood pressure (mmHg; ± s.e. mean) under the four treatment conditions.
| 22 °C | 40 °C | |||
|---|---|---|---|---|
| Placebo | Propranolol 40 mg | Placebo | Propranolol 40 mg | |
| Systolic BP | ||||
| Supine | 117.3 ± 2.4 | 112.4 ±2.3 | 115.4 ±1.6 | 112.2 ±2.9 |
| Erect | 115.6 ±3.4 | 113.8 ±2.3 | 109.4 ±3.9 | 110.7 ±3.1 |
| Diastolic BP | ||||
| Supine | 76.0 ±2.2 | 76.3 ±3.2 | 72.8 ±1.8 | 74.1 ±2.2 |
| Erect | 85.6 ±2.4 | 87.8 ±2.5 | 83.8 ±3.1 | 83.1 ±2.1 |
Physiological finger tremor
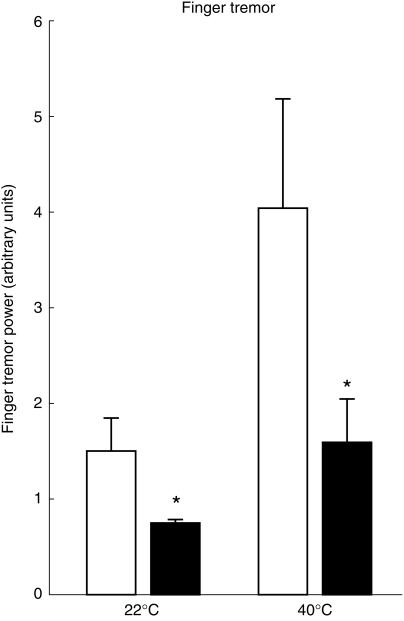
The effects of the treatment conditions on the power of physiological finger tremor are shown in Figure 3 and Table 1. Two-way anova revealed significant main effects of temperature condition (F1,11 = 9.5; P < 0.05) and drug treatment (F1,11 = 12.1; P < 0.01) and a significant interaction (F1,11 = 5.1; P < 0.05). Compared with the 22 °C condition, the 40 °C condition produced an increase in the power of physiological finger tremor, both in the presence of placebo (t = 6.6) and in the presence of propranolol (t = 2.0) (see Table 1).
Figure 3.
Power of physiological finger tremor. Open columns: placebo; closed columns: propranolol 40 mg. The height of each column corresponds to the mean (n = 12), vertical bars are s.e. mean. Statistically different differences from placebo *P < 0.05.
Discussion
In agreement with previous reports [2], there was a small increase in body temperature under the high ambient temperature condition, both in the presence of placebo and propranolol. The high temperature condition failed to have any effect on either systolic or diastolic blood pressure, consistent with earlier observations [2].
The high temperature condition caused an increase in the power of physiological finger tremor, confirming previous observations [2, 10, 11]: this may be attributed to sympathetic activation by the heat stressor [2]. Physiological finger tremor is believed to be mediated by β2-adrenoceptors in striated muscles [12–16], and it has been suggested that these receptors may be activated by circulating catecholamines [17]. The enhancement of physiological finger tremor by the heat stressor is likely to be due to sympathetic activation, since it has been shown that other variables associated with an increase in sympathetic activity (e.g. anxiety and thyrotoxicosis [18]) also lead to an increase in physiological finger tremor.
The high temperature condition increased heart rate, in agreement with previous reports [2, 19], and consistent with the sympathetic activating effect of high ambient temperature. However, an increase in heart rate may also be secondary to vagal withdrawal. Therefore, we have decided to examine whether there is any evidence for a change in the parasympathetic input to the heart, by studying the effect of the heat stressor on heart rate variability evoked by respiration (physiological sinus dysrhythmia) and change in posture from lying to standing (30 : 15 ratio). It is generally accepted that both the power of sinus dysrhythmia and the 30 : 15 ratio reflect parasympathetic activity. However, in order to examine the effect of the heat stressor on heart rate variability it was necessary to study these cardiac reflexes after the heart rate had been ‘reset’ to its level recorded at room temperature, since there is evidence that resting heart rate may affect cardiac reflexes [20–24]. Thus it has been shown that when there is an increase in heart rate due to β-adrenoceptor stimulation following the infusion of isoprenaline, there is an apparent reduction in parasympathetically mediated cardiac reflexes, indicating that β-adrenoceptor stimulation may mimic the effects of diminished parasympathetic function [23] and that the parasympathetic reflexes are ‘heart-rate-dependent’[7]. Therefore, it has been suggested that the parasympathetic cardiac reflexes should be studied under β-adrenoceptor blockade when any sympathetically mediated increase in heart rate has been removed [24].
In the present experiment, we used propranolol in an attempt to correct for the high-temperature-evoked increase in heart rate. Propranolol (40 mg) appeared to be effective in abolishing the effect of the heat stressor on resting heart rate: indeed, heart rate under the high temperature condition in the presence of propranolol was similar to that recorded at room temperature in the presence of placebo. Therefore we suggest that the high-temperature-induced reduction in heart rate variability was not simply a secondary effect of a change in baseline heart rate. The heat stressor significantly reduced both respiratory and postural heart rate variability as evidenced by reductions in the power of sinus dysrhythmia and the 30 : 15 ratio. As this effect of the heat stressor was also observable in the presence of propranolol when the heart rate was ‘reset’ to its room temperature level, we can conclude that the heat stressor was effective in attenuating the parasympathetic control of heart rate variability.
These results demonstrate the importance of the parasympathetic input to the heart in the control of cardiac function. It is of interest that while the heat stressor has been shown to cause a generalized enhancement of sympathetically mediated functions (e.g. cardiovascular activity [2]; pupillary function [4]; sweat gland activity [3]), only in the case of the heart is there evidence for an associated reduction in parasympathetic activity, as shown by the present results. Indeed, it has been shown that other parasympathetically mediated functions (e.g. the amplitude of the pupillary light reflex response [4]; salivary output [2]) remain unaffected by the high ambient temperature condition. These observations further illustrate the complex non-uniform organization of the autonomic control of different organs and functions [2, 25].
References
- 1.Bini G, Hagbarth KE, Hynniken P, Wallin BG. Regional similarities and differences in thermoregulatory motor and sudomotor tone. J Physiol. 1980;306:553–565. doi: 10.1113/jphysiol.1980.sp013414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arya DK, Langley RW, Szabadi E, Bradshaw CM. Comparison of the effects of high ambient temperature and clonidine on autonomic functions in man. Naunyn-Schmiedeberg's Arch Pharmacol. 1997;355:376–383. doi: 10.1007/pl00004957. [DOI] [PubMed] [Google Scholar]
- 3.Banjar W, Longmore J, Bradshaw CM, Szabadi E. The effect of diazepam on the responsiveness of human eccrine sweat glands to carbachol: influence of ambient temperature. Eur J Clin Pharmacol. 1987;31:661–665. doi: 10.1007/BF00541292. [DOI] [PubMed] [Google Scholar]
- 4.Leung NK-C, Bradshaw CM, Szabadi E. Effect of high ambient temperature on the kinetics of the pupillary light reflex in healthy volunteers. Br J Clin Pharmacol. 1992;33:458–460. doi: 10.1111/j.1365-2125.1992.tb04069.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smith SA. Reduced sinus arrhythmia in diabetic autonomic neuropathy: diagnostic value of an age related normal range. Br Med J. 1982;285:1599–1601. doi: 10.1136/bmj.285.6355.1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Borst C, Wieling W, van Brederode JFM, Hond A, De Rijk JG, Dunning AJ. Mechanisms of initial heart-rate response to postural change. Am J Physiol. 1982;244:767–781. doi: 10.1152/ajpheart.1982.243.5.H676. [DOI] [PubMed] [Google Scholar]
- 7.Piha SJ. Cardiovascular reflex tests and the resting heart rate at night and during testing. Clin Physiol. 1992;12:225–227. doi: 10.1111/j.1475-097x.1992.tb00308.x. [DOI] [PubMed] [Google Scholar]
- 8.Cruickshank JM, Pritchard BNC. Beta-Blockers in Clinical Practice. 2. Edinburgh: Churchill Livingstone; 1994. [Google Scholar]
- 9.Banjar WMA, Gazzaz J, Langley RW, Bradshaw CM, Szabadi E. Effects of high ambient temperature on parasympathetically-mediated cardiovascular reflexes in man. Br J Clin Pharmacol. 1999. p. 889P. [DOI] [PMC free article] [PubMed]
- 10.Al-Eithan M, Banjar WMA, Gazzaz J, Bradshaw CM, Szabadi E. Effects of high ambient temperature and terbutaline on physiological tremor. Br J Clin Pharmacol. 1991;31:603P. [Google Scholar]
- 11.Fleming PFJ, Lodwick J, Bradshaw CM, Szabadi E. Failure of clonidine to affect physiological tremor in healthy volunteers. Br J Clin Pharmacol. 1991;31:603P–604P. [Google Scholar]
- 12.Marsden CD, Foley TH, Owen DAL, McAllister RG. Peripheral beta-adrenergic receptors concerned with tremor. Clin Sci. 1967;33:53–65. [PubMed] [Google Scholar]
- 13.Marsden CD, Meadows JC, Lowe RD. The influence of nor-adrenaline, tyramine and activation of sympathetic nerves on physiological tremor in man. Clin Sci. 1969;37:243–252. [Google Scholar]
- 14.Larsson T, Svedmyr N. Tremor caused by sympathomimetics is mediated by beta-2 adrenoreceptors. Scand J Resp Dis. 1977;58:5–10. [PubMed] [Google Scholar]
- 15.Perucca E, Pickles H, Richens A. Effects of atenolol, metoprolol and propranolol on isoproterenol-induced tremor and tachycardia in normal subjects. Clin Pharmacol Ther. 1981;29:425–433. doi: 10.1038/clpt.1981.59. [DOI] [PubMed] [Google Scholar]
- 16.Abila B, Wilson JF, Marshall RW, Richens A. The tremorolytic actions of β2-adrenoceptor blockers in essential, physiological and isoprenaline-induced tremor is mediated by β2-adrenoceptors located in a deep peripheral compartment. Br J Clin Pharmacol. 1985;20:369–376. doi: 10.1111/j.1365-2125.1985.tb05079.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Young RR. Physiological and enhanced physiological tremor. In: Findley LJ, Capildeo R, editors. Movement Disorders: Tremor. London: Macmillan; 1984. pp. 85–93. [Google Scholar]
- 18.Marsden CD, Gimlett TMD, McAllister RG, Owen DAL, Miller TN. The effect of beta-adrenergic blockade on finger tremor and Achilles reflex time in anxious and thyrotoxic patients. Acta Endocrinol. 1968;57:353–362. doi: 10.1530/acta.0.0570353. [DOI] [PubMed] [Google Scholar]
- 19.Kukkonen-Harjula K, Oja P, Vuori I, et al. Cardiovascular effects of atenolol, scopolamine and their combination on healthy men in Finnish sauna baths. J Appl Physiol. 1994;69:10–15. doi: 10.1007/BF00867920. [DOI] [PubMed] [Google Scholar]
- 20.Bennett T, Farquhar IK, Hosking DJ, Hampton JR. Assessment of methods for estimating autonomic nervous control of the heart in patients with diabetes mellitus. Diabetes. 1978;27:1167–1174. doi: 10.2337/diab.27.12.1167. [DOI] [PubMed] [Google Scholar]
- 21.Smith SE, Smith SA. Heart-rate variability in healthy subjects measured with a bedside computer-based technique. Clin Sci. 1981;61:379–383. doi: 10.1042/cs0610379. [DOI] [PubMed] [Google Scholar]
- 22.Piha SJ. Cardiovascular autonomic reflex tests: normal responses and age-related reference values. Clin Physiol. 1991;11:277–290. doi: 10.1111/j.1475-097x.1991.tb00459.x. [DOI] [PubMed] [Google Scholar]
- 23.Pfeifer MA, Cook D, Brodsky J, et al. Quantitative evaluation of cardiac parasympathetic activity in normal and diabetic man. Diabetes. 1982;31:339–345. doi: 10.2337/diab.31.4.339. [DOI] [PubMed] [Google Scholar]
- 24.Pfeifer MA, Weinberg CR, Cook D, Best JD, Reenan A, Halter JB. Differential changes of autonomic nervous system function with age in man. Am J Med. 1983;75:249–258. doi: 10.1016/0002-9343(83)91201-9. [DOI] [PubMed] [Google Scholar]
- 25.Szabadi E, Bradshaw CM. Autonomic pharmacology of α2-adrenoceptors. J Psychopharmacol. 1996;10(Suppl 3):6–18. [Google Scholar]