Abstract
Aims
To study the effects of rifampicin on the pharmacokinetics and pharmaco-dynamics of glimepiride, a new sulphonylurea antidiabetic drug.
Methods
In this randomised, two-phase cross-over study, 10 healthy volunteers were treated for 5 days with 600 mg rifampicin or placebo once daily. On day 6, a single oral dose of 1 mg glimepiride was administered. Plasma glimepiride and blood glucose concentrations were measured up to 12 h.
Results
Rifampicin decreased the mean area under the plasma concentration-time curve of glimepiride by 34% (P < 0.001) and the mean elimination half-life by 25% (P < 0.05). No significant differences in the blood glucose response to glimepiride were observed between the placebo and rifampicin phases. However, symptomatic hypoglycaemia occurred only during the placebo phase.
Conclusions
The effects of rifampicin on the pharmacokinetics of glimepiride suggest that rifampicin induced the CYP2C9-mediated metabolism of glimepiride and thereby slightly increased its systemic clearance. Because the interaction was modest and did not significantly alter the glucose-lowering effect of glimepiride in healthy volunteers, it is probably of limited clinical significance. However, in some patients the hypoglycaemic effect of glimepiride may be reduced during concomitant treatment with rifampicin.
Keywords: CYP2C9, glimepiride, interaction, rifampicin
Introduction
Glimepiride is a new sulphonylurea antidiabetic agent. It is completely absorbed after oral administration and is eliminated mainly via metabolism by CYP2C9 [1]. The oral bioavailability of glimepiride is close to 100% [1].
Rifampicin, a rifamycin antibiotic, is a potent inducer of cytochrome P450 enzymes and can greatly reduce the plasma concentrations and effects of numerous drugs [2–6]. However, there seem to be no published studies on the effects of enzyme induction on the pharmacokinetics of glimepiride. Because rifampicin can induce CYP2C9-mediated drug metabolism and glimepiride is a substrate of CYP2C9 [1], it is important to study the possible effects of rifampicin on the pharmacokinetics and pharmacodynamics of glimepiride.
Methods
Subjects
Ten healthy volunteers (five men and five women; age range 19–26 years; weight range 52–73 kg) participated in the study after giving written informed consent (Table 1). There were no dropouts. The volunteers were ascertained to be healthy by a medical history, a physical examination, and routine blood chemistry tests before entering the study. All five women were using oral contraceptive steroids and were advised to use other methods for contraception starting 1 week before the first premedication day and continuing until the end of the first full menstrual cycle after the study. None of the subjects had other continuous medication.
Table 1.
Characteristics of the subjects.
| Subject | Sex | Age (years) | Weight (kg) | Smoker | Use of oral contraceptives |
|---|---|---|---|---|---|
| 1 | Male | 23 | 73 | No | No |
| 2 | Male | 22 | 65 | No | No |
| 3 | Female | 25 | 53 | No | 30 µg ethinylestradiol + 75 µg gestodene |
| 4 | Female | 25 | 65 | No | 30 µg ethinylestradiol + 75 µg gestodene |
| 5 | Female | 25 | 63 | No | 20 µg ethinylestradiol + 0.15 mg desogestrel |
| 6 | Female | 24 | 52 | Yes | 30 µg levonorgestrel |
| 7 | Male | 21 | 67 | No | No |
| 8 | Male | 23 | 67 | No | No |
| 9 | Male | 26 | 72 | Yes | No |
| 10 | Female | 19 | 55 | Yes | 30 µg ethinylestradiol + 75 µg gestodene |
Study design
The study protocol was approved by the Ethics Committee of the Department of Clinical Pharmacology, University of Helsinki, and the Finnish National Agency for Medicines. A randomised crossover study design with two phases was used. The phases were separated by a 4 week washout period. The volunteers took either 600 mg rifampicin (one Rifarm 600 mg tablet divided into two capsules, Pharmacal, Helsinki, Finland) or matched placebo orally once daily at 20.00 h for 5 days. On day 6, after an overnight fast, a single dose of 1 mg glimepiride (one Amaryl 1 mg tablet, Hoechst Marion Roussel, Stockholm, Sweden) was administered orally with 150 ml water at 08.30 h, that is, 12.5 h after the last dose of rifampicin. Glimepiride was ingested in sitting position, and the subjects spent the first 3 h seated. Blood glucose concentrations were monitored throughout the day. Glucose for both oral and intravenous use and glucagon for intramuscular use were available in case of severe hypoglycaemia, but they were not needed.
Meals
The volunteers received a standard light breakfast precisely 15 min after the ingestion of glimepiride, a standard warm meal after 3 h and a standard light meal after 7 h. Food intake was identical during both days of glimepiride administration. The breakfast was eaten within 10 min and contained approximately 370 kcal energy, 70 g carbohydrates, 8 g protein and 6 g fat.
Blood sampling and determination of blood glucose concentrations
On the days of administration of glimepiride, a forearm vein of each subject was cannulated with a plastic cannula and kept patent with an obturator. Timed blood samples were drawn just before glimepiride administration and 0.5, 1, 1.5, 2, 2.5, 3, 4, 5, 7, 9 and 12 h later. The blood samples (10 ml each) were taken into tubes that contained ethylenediaminetetraacetic acid (EDTA). Blood glucose was measured immediately after each blood sampling by the glucose oxidase method with the Precision G Blood Glucose Testing System (MediSense, An Abbot Laboratories Company, Bedford, USA) that uses an electrochemical detection technique. Blood glucose concentrations were measured up to 9 h. Plasma was separated after the determination of blood glucose concentration and stored at −40 °C until analysis.
Determination of plasma glimepiride concentrations
Plasma glimepiride concentrations were quantified by liquid chromatography-mass spectrometry with use of the PE SCIEX API 3000 LC/MS/MS System (Sciex Division of MDS Inc, Toronto, Canada), with glibenclamide as the internal standard. A Hypersil BDS-C18 column and a mobile phase consisting of acetonitrile (57%) and 10 mm ammonium formate (pH 3.5; 43%) were used. The ion transitions monitored were m/z 491 to m/z 352 for glimepiride and m/z 494 to m/z 369 for glibenclamide. These transitions represent the product ions of the [M + H]+ ions. The limit of quantification of the method was 0.1 ng ml−1 and the interday coefficient of variation was 6.8% at 0.1 ng ml−1, 7.5% at 0.6 ng ml−1, 5.7% at 6.4 ng ml−1, 3.7% at 54.1 ng ml−1 and 2.0% at 98.7 ng ml−1 (n = 4).
Pharmacokinetics of glimepiride
The pharmacokinetics of glimepiride were characterized by peak concentration in plasma (Cmax), concentration peak time (tmax), elimination half-life (t½) and area under the concentration-time curve (AUC). The terminal log-linear part of the concentration-time curve was identified visually for each subject. The elimination rate constant (kel) was determined by linear regression analysis of the log-linear part of the plasma drug concentration-time curve. The t½ was calculated by the equation t½ = ln2/kel. The AUC was calculated by use of the linear trapezoidal rule with extrapolation to infinity by dividing the last measured concentration by kel.
Glucose response
Glucose response was characterized by determining the decremental area under the blood glucose concentration-time curve from 0 to 3 h [AUC(0,3 h)] and 0 to 7 h [AUC(0,7 h)]. The maximal increase in blood glucose concentration after the breakfast and the maximal decrease compared with the baseline value (before glimepiride administration) were calculated during the first 3 h after the administration of glimepiride.
Statistical analysis
Results are expressed as mean values±s.e. mean. 95% confidence intervals (CI) on the mean differences between the placebo and rifampicin phases were calculated for all variables except tmax. The pharmacokinetic variables and the glucose response data after the two pretreatments were compared with a paired t-test (two-tailed) or, in the case of tmax, by the Wilcoxon signed-rank test. Before statistical analysis, the pharmacokinetic variables (except for tmax) were log transformed. The level of statistical significance was P < 0.05.
Results
Pharmacokinetics of glimepiride
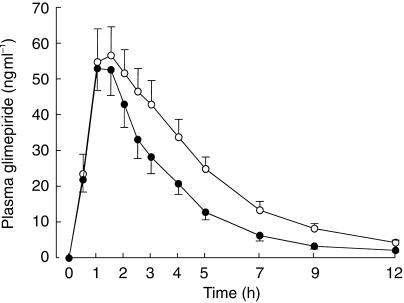
Pretreatment with rifampicin moderately decreased plasma glimepiride concentrations (Figure 1 and Table 2). The mean AUC of glimepiride was reduced by 34% (P < 0.001) by rifampicin. A reduction in the AUC (range, 14% to 41%) was seen in each of the subjects (Figure 2). The mean Cmax of glimepiride was decreased by 14% (P = 0.06) and the mean t½ was shortened from 2.6±0.3 h to 2.0±0.2 h (P < 0.05) by rifampicin.
Figure 1.
Mean (s.e. mean) plasma concentrations of glimepiride in 10 healthy volunteers after a single 1 mg oral dose of glimepiride, following a 5 day pretreatment with placebo (open circles) or 600 mg rifampicin (solid circles) once daily.
Table 2.
The pharmacokinetic variables of 1 mg oral glimepiride in 10 healthy subjects after pretreatment with placebo or 600 mg rifampicin once daily for 5 days.
| Variable | Placebo phase (control) | Rifampicin phase | Mean difference between placebo and rifampicin (95% CI) |
|---|---|---|---|
| Cmax (ng ml−1) | 64.2±9.1 | 55.5±7.2 | 8.7 (−0.60, 18.10) |
| tmax (h) | 1.5±(1.0–3.0) | 1.0±(1.0–2.0) | |
| t½ (h) | 2.6±0.3 | 2.0±0.2* | 0.7 (−0.1, 1.4) |
| AUC (ng ml−1 h) | 286.7±35.1 | 190.3±25.2† | 96.4 (68.8, 124.0) |
Data are mean values±s.e. mean; tmax data are given as median with range.
Significantly (P < 0.05) different from the placebo phase.
Significantly (P ≤0.001) different from the placebo phase.
Figure 2.
Individual AUC values of glimepiride in 10 healthy volunteers after a single 1 mg oral dose of glimepiride, following a 5 day pretreatment with placebo or 600 mg rifampicin once daily. The broken lines indicate the five female subjects, who were all using oral contraceptive steroids.
Effect on blood glucose concentrations
No statistically significant differences were observed in the blood glucose response to glimepiride between the placebo and rifampicin phases (Figure 3 and Table 3). The lowest observed blood glucose concentration, 2.5 mm, occurred in subjects 7 and 9 during the placebo phase and was associated with mild symptoms of hypoglycaemia only. No other adverse events occurred during the study.
Figure 3.
Blood glucose concentrations (mean±s.e. mean) in 10 healthy volunteers after a single 1 mg oral dose of glimepiride, following a 5 day pretreatment with placebo (open circles) or 600 mg rifampicin (solid circles) once daily. A standard breakfast was served 15 min after the administration of glimepiride and a standard warm meal after 3 h.
Table 3.
The blood glucose response to 1 mg oral glimepiride in 10 healthy subjects after pretreatment with placebo or 600 mg rifampicin once daily for 5 days. A standard breakfast was served 15 min after the administration of glimepiride and a standard warm meal after 3 h.
| Variable | Placebo phase (control) | Rifampicin phase | Mean difference between placebo and rifampicin (95% CI) |
|---|---|---|---|
| Blood glucose | |||
| Decremental AUC(0,3 h) (mm h) | 0.57±0.50 | 0.26±0.55 | 0.31 (−0.49, 1.11) |
| Decremental AUC(0,7 h) (mm h) | 4.51±1.07 | 5.05±1.34 | −0.54 (−2.62, 1.54) |
| Maximal increase (mm) | 1.6±0.4 | 2.2±0.4 | −0.6 (−1.5, 0.3) |
| Maximal decrease (mm) | 1.7±0.1 | 1.7±0.2 | 0.0 (−0.5, 0.5) |
Data are mean±values s.e. mean; maximal increase and maximal decrease refer to the maximal increase and decrease in blood glucose concentration during the first 3 h after glimepiride administration.
Discussion
In this study the plasma concentrations of glimepiride were moderately decreased by pretreatment with rifampicin. The mean AUC and t½ of glimepiride were decreased by 34% and 25% by rifampicin, respectively. However, no statistically significant differences in the blood glucose response to glimepiride administration were observed between the placebo and rifampicin phases. There was considerable interindividual variation in the extent of the rifampicin–glimepiride interaction, the decrease in the glimepiride AUC ranging from 14% to 41% even in this homogenous group of young healthy volunteers. It is possible that variation in the extent of this interaction is larger in elderly patients who are the real target population of glimepiride and who may exhibit additional factors affecting this interaction.
Glimepiride is metabolized in the liver by CYP2C9 to a hydroxy metabolite, which is further dehydrogenated by the cytosolic alcohol and aldehyde dehydrogenase enzymes to a carboxy metabolite [1]. The mechanism underlying the interaction between rifampicin and glimepiride is probably induction of the CYP2C9-mediated biotransformation of glimepiride by rifampicin. Because rifampicin moderately decreased the t½ of glimepiride and only slightly decreased the Cmax, it seems that rifampicin induced glimepiride metabolism mainly during the elimination phase and thereby slightly increased its systemic clearance. As a low clearance drug [1], glimepiride is not susceptible to marked metabolic drug interactions during the first-pass, as was seen in this study. The observation that the reduction of plasma glimepiride concentrations did not result in a significant reduction in the glucose response may be explained by the relatively low magnitude of the pharmacokinetic interaction and by a weak dose–response relationship, as seen with glimepiride in 1–8 mg doses [1].
In previous studies with healthy subjects, rifampicin has been shown to induce the metabolism of CYP2C9 substrates other than glimepiride. For example, the interaction between rifampicin and the CYP2C9 substrate S-warfarin is well known [7, 8]. Furthermore, the coadministration of rifampicin with losartan resulted in a 35% reduction in the AUC(0,24 h) of losartan and a 40% reduction in the AUC(0,24 h) of the losartan metabolite E3174 [9]. The interactions of rifampicin with many ‘pure’ CYP3A4 substrates are of a much greater magnitude than those occurring with CYP2C9 substrates. For example, a 5 day pretreatment with 600 mg rifampicin daily reduced the mean AUC of oral midazolam, triazolam and buspirone to 4%, 5% and 10% of the control value, respectively [3, 5, 6].
Rifampicin induces the metabolism of tolbutamide, a first generation sulphonylurea and a model substrate of CYP2C9 [10], as demonstrated by the 43% reduction in tolbutamide half-life observed in one study [11]. Reports on the effects of rifampicin on second generation sulphonylureas are sparse. In a recent study, the blood glucose lowering effect of glibenclamide was significantly reduced by rifampicin [12]. Unfortunately, no pharmacokinetic data were obtained in this study.
Because the magnitude of the interaction of rifampicin with glimepiride was modest and the blood glucose response to glimepiride was not affected in healthy volunteers, this interaction is probably of limited clinical significance only. However, because of the considerable interindividual variation in the extent of the interaction, rifampicin may reduce the blood glucose lowering effect of glimepiride in some patients. It is therefore advisable to closely monitor blood glucose levels if rifampicin is added to the therapy of a diabetic patient treated with glimepiride or if rifampicin treatment is discontinued.
In conclusion, rifampicin modestly reduced the plasma concentrations of glimepiride in healthy volunteers, probably by inducing the CYP2C9-mediated metabolism of glimepiride during the elimination phase. Rifampicin did not have a significant effect on the blood glucose response to glimepiride administration.
Acknowledgments
We would like to thank Mr Jouko Laitila, Mr Mikko Neuvonen and Mrs Kerttu Mårtensson for the skilful determination of plasma glimepiride concentrations. This study was supported by grants from the Helsinki University Central Hospital Research Fund and the National Technology Agency of Finland (TEKES).
References
- 1.Langtry HD, Balfour JA. Glimepiride. A review of its use in the management of type 2 diabetes mellitus. Drugs. 1998;55:563–584. doi: 10.2165/00003495-199855040-00007. [DOI] [PubMed] [Google Scholar]
- 2.Venkatesan K. Pharmacokinetic drug interactions with rifampicin. Clin Pharmacokin. 1992;22:47–65. doi: 10.2165/00003088-199222010-00005. [DOI] [PubMed] [Google Scholar]
- 3.Backman JT, Olkkola KT, Neuvonen PJ. Rifampin drastically reduces plasma concentrations and effects of oral midazolam. Clin Pharmacol Ther. 1996;59:7–13. doi: 10.1016/S0009-9236(96)90018-1. [DOI] [PubMed] [Google Scholar]
- 4.Strayhorn VA, Baciewicz AM, Self TH. Update on rifampin drug interactions III. Arch Intern Med. 1997;157:2453–2458. [PubMed] [Google Scholar]
- 5.Villikka K, Kivistö KT, Backman JT, Olkkola KT, Neuvonen PJ. Triazolam is ineffective in patients taking rifampin. Clin Pharmacol Ther. 1997;61:8–14. doi: 10.1016/S0009-9236(97)90176-4. [DOI] [PubMed] [Google Scholar]
- 6.Lamberg TS, Kivistö KT, Neuvonen PJ. Concentrations and effects of buspirone are considerably reduced by rifampicin. Br J Clin Pharmacol. 1998;45:381–385. doi: 10.1046/j.1365-2125.1998.t01-1-00698.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heimark LD, Gibaldi M, Trager WF, O'reilly RA, Goulart DA. The mechanism of the warfarin–rifampin drug interaction in humans. Clin Pharmacol Ther. 1987;42:388–394. doi: 10.1038/clpt.1987.168. [DOI] [PubMed] [Google Scholar]
- 8.Kaminsky LS, Zhang ZY. Human P450 metabolism of warfarin. Pharmacol Ther. 1997;73:67–74. doi: 10.1016/s0163-7258(96)00140-4. 10.1016/s0163-7258(96)00140-4. [DOI] [PubMed] [Google Scholar]
- 9.Williamson KM, Patterson JH, McQueen RH, Adams Kf, Jr, Pieper JA. Effects of erythromycin or rifampin on losartan pharmacokinetics in healthy volunteers. Clin Pharmacol Ther. 1998;63:316–323. doi: 10.1016/S0009-9236(98)90163-1. [DOI] [PubMed] [Google Scholar]
- 10.Srivastava PK, Yun CH, Beaune PH, Ged C, Guengerich FP. Separation of human liver microsomal tolbutamide hydroxylase and (S) -mephenytoin 4′-hydroxylase cytochrome P-450 enzymes. Mol Pharmacol. 1991;40:69–79. [PubMed] [Google Scholar]
- 11.Syvälahti E, Pihlajamäki K, Iisalo E. Effect of tuberculostatic agents on the response of serum growth hormone and immunoreactive insulin to intravenous tolbutamide, and on the half-life of tolbutamide. Int J Clin Pharmacol Biopharm. 1976;13:83–89. [PubMed] [Google Scholar]
- 12.Surekha V, Peter JV, Jeyaseelan L, Cherian AM. Drug interaction: rifampicin and glibenclamide. Nat Med J India. 1997;10:11–12. [PubMed] [Google Scholar]