Abstract
Aims
The role of flavin containing monooxygenases (FMO) on the disposition of many drugs has been insufficiently explored. In vitro and in vivo tests are required to study FMO activity in humans. Benzydamine (BZD) N-oxidation was evaluated as an index reaction for FMO as was the impact of genetic polymorphisms of FMO3 on activity.
Methods
BZD was incubated with human liver microsomes (HLM) and recombinant enzymes. Human liver samples were genotyped using PCR-RFLP.
Results
BZD N-oxide formation rates in HLM followed Michaelis-Menten kinetics (mean Km = 64.0 μm, mean Vmax = 6.9 nmol mg−1 protein min−1; n = 35). N-benzylimidazole, a nonspecific CYP inhibitor, and various CYP isoform selective inhibitors did not affect BZD N-oxidation. In contrast, formation of BZD N-oxide was almost abolished by heat treatment of microsomes in the absence of NADPH and strongly inhibited by methimazole, a competitive FMO inhibitor. Recombinant FMO3 and FMO1 (which is not expressed in human liver), but not FMO5, showed BZD N-oxidase activity. Respective Km values for FMO3 and FMO1 were 40.4 μm and 23.6 μm, and respective Vmax values for FMO3 and FMO1 were 29.1 and 40.8 nmol mg−1 protein min−1. Human liver samples (n = 35) were analysed for six known FMO3 polymorphisms. The variants I66M, P135L and E305X were not detected. Samples homozygous for the K158 variant showed significantly reduced vmax values (median 2.7 nmol mg−1 protein min−1) compared to the carriers of at least one wild type allele (median 6.2 nmol mg−1 protein min−1) (P<0.05, Mann–Whitney- U-test). The V257M and E308G substitutions had no effect on enzyme activity.
Conclusions
BZD N-oxidation in human liver is mainly catalysed by FMO3 and enzyme activity is affected by FMO3 genotype. BZD may be used as a model substrate for human liver FMO3 activity in vitro and may be further developed as an in vivo probe reflecting FMO3 activity.
Keywords: flavin-containing monooxygenase, benzydamine N-oxidation, pheno-typing, human
Introduction
Flavin-containing monooxygenases (FMO) contribute to the biotransformation of many xenobiotics and display a broad substrate spectrum. The mechanistic and kinetic properties of these enzymes differ from those of the cytochrome P-450 (CYP) family and enable FMO to oxygenate most soft nucleophiles, i.e. compounds carrying nitrogen or sulphur in functional groups [1, 2]. Substrates include dietary compounds as well as drugs, such as alkaloids, antihistamines, phenothiazines and tricyclic antidepressants [3, 4]. The human FMO isoforms, commonly denoted FMO1 through FMO5 [5, 6], differ in tissue distribution and have different but often overlapping substrate specificities [3].
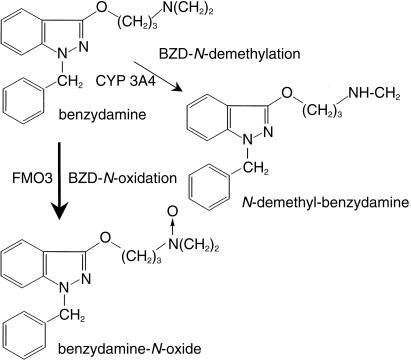
FMO3 appears to be the major isoform expressed in human liver [7, 8]. Sequence analysis [9] of the FMO3 gene has revealed several polymorphisms. Three mutations are known to cause the metabolic disorder trimethylami-nuria (‘fish odour syndrome’) [10–13], while other amino acid variants cause less substantial alterations in enzyme activity or have no effect [13]. Variability in drug response and clearance may be in part related to interindividual differences in FMO activity, similar to those described for various CYP isoforms. Consequently, convenient and reliable methods are required to determine FMO3 activity in vitro and in vivo. The measurement of trimethylamine metabolic parameters is a well established method for this purpose [14]. However, since exposure to this compound occurs from endogenous and exogenous sources, partial metabolic clearances via FMO3 cannot be precisely measured using trimethylamine-N-oxidation. Benzyda-mine (BZD) is a nonsteroidal antiinflammatory drug extensively metabolized to benzydamine N-oxide (BZD-NO) (Figure 1), the major metabolite found in plasma and urine of human subjects [15–17]. Previous studies demonstrated that N-oxidation is the major pathway of BZD biotransformation in various species in vitro and in situ[18, 19]. In rat liver microsomes BZD N-oxidation was shown to be catalysed by FMO whith a 2000-fold higher Vmax and 50-fold lower Km values than those reported for the CYP mediated formation of N-desmethyl-BZD (Figure 1) [18].
Figure 1.
Structure of benzydamine and pathways of benzydamine metabolism. Benzydamine-iV-demethylation is a minor pathway compared with benzydamine-N-oxidation.
The present study aimed to evaluate benzydamine-N-oxidation as an index reaction for human FMO activity in vitro. We employed human liver microsomes, chemical inhibitors and recombinant enzymes to elucidate the role of FMO in BZD N-oxidation and to investigate isoform specificity. Enzyme kinetic parameters were determined and the impact of genetic polymorphism of FMO3 on enzyme activity in vitro was assessed. The prospect of using BZD as a phenotyping probe in humans is discussed.
Methods
Chemicals
Benzydamine (1-benzyl-(3[3-dimethylamino]-propoxy)-1H-indazole) hydrochloride was kindly provided by Solvay Pharma (Hannover, Germany). Benzydamine N-oxide and N-desmethylbenzydamine were a generous gift from Dr Takabatake (Setsunan University, Osaka, Japan). Quinidine, sulphaphenazole, α-naphthoflavone, methi-mazole and diethyldithiocarbamate were purchased from Sigma (Deisenhofen, Germany), as were NADP, isoci-trate, isocitrate dehydrogenase and KH2PO4. Ketoconazole was obtained from PJ3I (Natick, MA, USA) and N-benzylimidazole from Lancaster (Miihlheim, Germany). MgCl2, Tris and all organic solvents (reagent grade) were purchased from Merck (Darmstadt, Germany). Omepra-zole was kindly provided by Astra (Wedel, Germany).
Liver samples and microsome preparation
Liver tissue from 35 Caucasian subjects was obtained from the International Institute for the Advancement of Medicine (Exton, PA, USA). The tissue was kept at −80°C until time of microsome preparation. Microsomes were prepared as described previously [20] and micro-somal protein content was determined using the bicinch-oninic acid protein assay (Pierce, Rockford, IL, USA) with bovine serum albumin as a standard.
Recombinant enzymes
Microsomes from baculovirus infected insect cells expressing FMO3, FMO1, FMO5, CYP3A4, CYP2C19, CYP2C9, CYP2D6, CYP1A2, CYP1E2 and CYP2A6 (supersomes™) were obtained from Gentest Corp. (Woburn, MA, USA).
Incubations
Enzyme kinetics and chemical inhibition
Methanolic solutions of substrates (2.5−500 μm BZD) and inhibitors were evaporated to dryness under mild vacuum at room temperature prior to addition of buffer and cofactors. Incubation mixtures contained 0.05 m KH2PO4 buffer (pH 7.4 at 25°C), 0.5 mm NADP, 3.75 mm (+)-isocitric acid, 1 U ml−1 of isocitrate dehydrogenase and 5 mm Mg2+. Although in vitro studies on FMO activity are commonly conducted at pH >8.0 to assure maximal FMO activity [18, 21], we applied a pH of 7.4 to reflect the in vivo situation more closely. The final volume was 250 μl, with a microsomal protein concentration of 100 μg ml−1. Inhibitors, substrate, incubation buffer and cofactor were preincubated at 37°C for 5 min, and reactions were initiated by addition of microsomes, except for incubations with diethyldithiocarbamate, which was preincubated with buffer, cofactors and microsomes for 20 min at 37°C before initiation of the reaction by addition of substrate. Samples were incubated for 20 min at 37°C, and reactions were terminated by addition of 100 μl acetonitrile and cooling on ice. Internal standard was added, samples were spun at 16000 g for 5 min and supernatants were analysed by h.p.l.c. Incubation mixtures with cDNA expressed enzymes were prepared with protein concentrations of 10 μg ml−1 (FMO3, FMO1) or 100 μg ml−1(FMO5).
Heat inactivation
To investigate FMO contribution to metabolite formation, duplicate sets of samples containing only buffer and microsomes were prepared. One set was supplemented with the NADPH generating system and both sets were incubated at 45°C for 5 min and then placed on ice. The NADPH generating system was added to that set of samples from which it was omitted previously. Substrate was added and both sets of samples were incubated under regular conditions. Control incubations were not heat treated [21].
Incubations were performed in duplicate, except for those using recombinant enzymes. Identity of metabolites was verified by comparing the retention times to those of authentic standards. Substrates and inhibitors were soluble in buffer over the concentration ranges used. Incubation times and microsomal protein concentrations were within the linear range of metabolite formation.
H.p.l.c. conditions
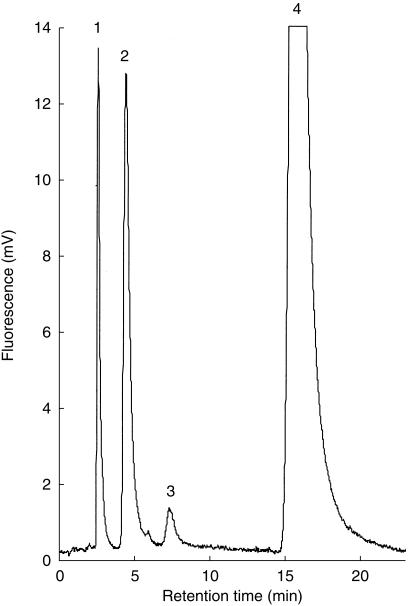
BZD, BZD-NO and N-desmethyl-BZD were separated using a 4.0 × 125 mm 5 μm Optilabsphere™ ODS2 (C18) column (VDS Optilab) at 55°C. A Shimadzu RF 551 fluorimetric detecor (Kyoto, Japan) was set at 308/ 350 nm (excitation/emmission). The eluent consisted of 32% acetonitrile and 68% 0.05 m KH2PO4, pH 7.0 and was delivered at 1.4 ml min−1 which was increased to 1.8 ml min−1 after 7 min. Retention times were 2.5 min for talinolol (internal standard), 4.4 min for BZD-NO, 7.1 min for N-desmethyl-BZD and 15.9 min for BZD (Figure 2). Compounds were quantified using the internal standard method based on peak height. The detection limit for BZD-NO was 0.02 ng injected. Inhibitors did not interfere with the assay. The intra-assay coefficient of variation (CV) for BZD-NO formation rates at 5 μm BZD was 5.5% (n = 6), and the between day CV was 12.8% (n = 8). Samples were stable at room temperature for 7 days (CV 5.4%). The mean CV for duplicate incubations with human liver microsomes was 6.9%.
Figure 2.
Figure 2 H.p.l.c. trace of an incubation of 50 μm benzydamine with human liver microsomes using fluorimetric detection at 308/350 nm (excitation/emission). Peaks are identified as follows: 1: Talinolol (internal standard),2: Benzydamine-N-oxide,3: N-desmethylbenzydamine,4: Benzydamine.
FMO3 genotyping
For preparation of genomic DNA, 1 g of homogenized liver tissue was digested with Proteinase K and subjected to a three step phenol/chloroform extraction. DNA was dissolved in 10 mm Tris, 1 mm EDTA, pH 8.0 and stored at 4°C. Liver samples were analysed for six known polymorphisms using PCR-RFLP methods described previously [10, 22] (Table 1).
Table 1.
FMO3 polymorphisms: locations and methods of PCR-RFLP analysis.
| Amino acid change | Point mutationa | Location | Detection | PCR. conditions |
|---|---|---|---|---|
| M66I | 11192G>T | exon 3 | FokI | Sachse et al.[22] |
| P153L | 15153C>T | exon 4 | BamHl | Dolphin et al.[10] |
| E158K | 15167G>A | exon 4 | HinfI | Dolphin et al.[10] |
| V257M | 18281G>A | exon 6 | NlaIII | Sachse et al.[22] |
| E305X | 21433G>T | exon 7 | EcoRI | Sachse et al.[22] |
| E308G | 21443A>G | exon 7 | Bsp120I | Sachse et al.[22] |
nucleotide positions refer to GenBank sequence AL021026, the A of the start codon ATG was counted as nucleotide 1
Data analysis
Enzyme kinetic parameters were determined by nonlinear least square regression (SigmaPlot 4.01, SPSS Inc., Chicago, IL, USA) using the Michaelis-Menten equation. Goodness of fit was assessed by plots of residuals vs predicted values, plots of reaction velocity vs substrate concentration and Eadie-Hofstee plots [23]. The impact of genotype on enzyme activity was assessed using the Mann-Whitney-U-test and the Kruskall-Wallis test (SPSS 8.01, SPSS Inc., Chicago, IL, USA).
Results
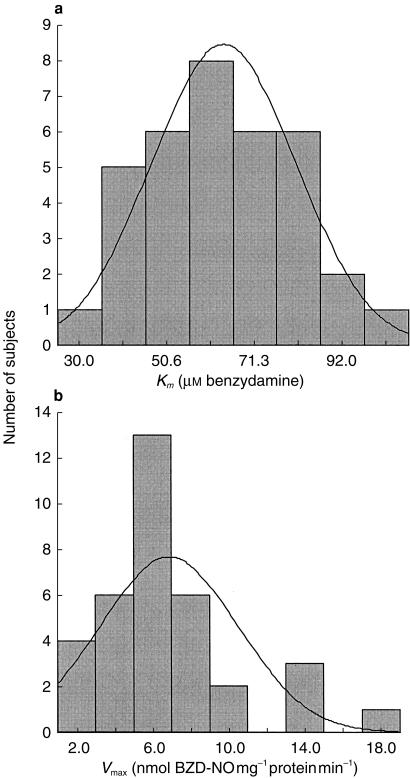
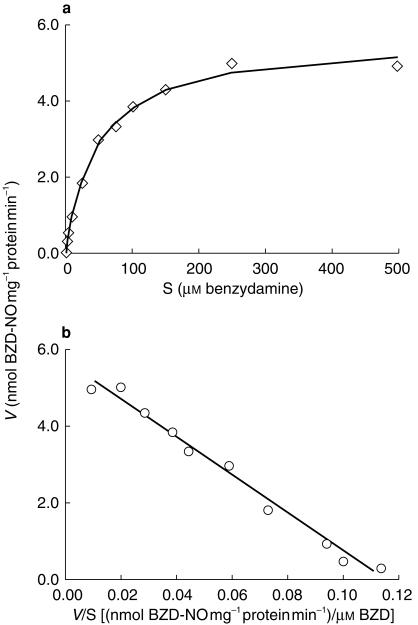
All 35 human liver samples catalysed extensive formation of BZD-NO, and Km values showed a four fold variation ranging from 28 to 102 μm (mean 64.0 μm, s.d. 17.0) (Table 2 and Figure 3). vmax values varied about 10-fold, ranging from 1.5 to 17.6 nmol mg−1 protein min−1 (mean 6.9 nmol mg−1 protein min−1, s.d. 3.6) (Table 2 and Figure 3). Formation of BZD-NO from BZD by human liver microsomes was consistent with a single enzyme Michaelis-Menten model (Figure 4).
Table 2.
Kinetic parameters for benzydamine-N-oxidation by human liver microsomes and cDNA expressed FMO3, FMO1 and FMO5; Impact of genotype on enzyme activity in vitro.
| Kma | Vmaxb | ||||||
|---|---|---|---|---|---|---|---|
| HLM group | n | mean | median | 25 —15 percentile | mean | median | 25 —15 percentile |
| Total | 35 | 64.0 | 58.7 | 52.4–77.3 | 6.9 | 6.5 | 4.1–7.8 |
| EE158 | 14 | 65.3 | 72.2 | 49.0–82.3 | 7.3 | 6.9 | 5.7–7.9 |
| EK158 | 16 | 64.4 | 57.6 | 54.4–80.7 | 7.0 | 6.5 | 5.1–7.6 |
| KK158 | 5 | 58.7 | 57.2 | 54.7–63.4 | 5.0 | 2.7 | 2.3–6.3*) |
| VV257 | 29 | 62.2 | 57.8 | 51.6–75.5 | 6.5 | 6.3 | 3.9–7.3 |
| VM257 | 4 | 66.0 | 65.1 | 52.4–80.6 | 10.1 | 10.5 | 6.2–13.5 |
| MM257 | 2 | 84.8 | 84.8 | 82.3–87.3 | 5.5 | 5.5 | 3.4–7.6 |
| EE308 | 24 | 66.1 | 69.6 | 51.9–82.2 | 7.2 | 6.5 | 5.2–7.8 |
| EG308 | 11 | 59.4 | 57.2 | 52.4–62.6 | 6.1 | 5.7 | 2.8–7.8 |
| GG308 | 0 | ||||||
| Recombinant | |||||||
| FMO3 | 40.0 | 29.0 | |||||
| FMO1 | 24.0 | 41.0 | |||||
| FMO5 | no activity | no activity | |||||
*) vmax significantly reduced in homozygous samples (KK158) compared with those carrying at least one wild type allele (EE158 + EK158); P<0.05, Mann-Whitney-U-test. (Kruskal-Wallis-test for all three groups: P = 0.104, Chi square = 4.519, d.f. = 2) a: Km[μm] -Michaelis-Menten constant; b: Vmax [nmol mg−1 protein min−1].
Figure 3.
Histograms showing the distribution of kinetic parameters for human liver microsomal benzydamine N- oxidation. a) Km values (n = 35, mean 64.0 μm, s.d. 17.0) and b) Vmax values (n = 35, mean 6.9 nmol mg∼ protein min∼, s.d. 3.6).
Figure 4.
Formation of BZD-N-oxide by human liver microsomes (HL014). a)plot of reaction velocity vs substrate concentration (Vvs S); b) Eadie-Hofstee plot (Vvs V/S). Symbols represent experimental points (means of duplicate samples), lines represent formation rates predicted with nonlinear regression and the Michaelis-Menten equation. Kinetic parameters: Km = 49 μm, Vmax = 5.7 nmol mg−1 protein min−1. HL014 is carrying the wild type allele of all six polymorphisms tested.
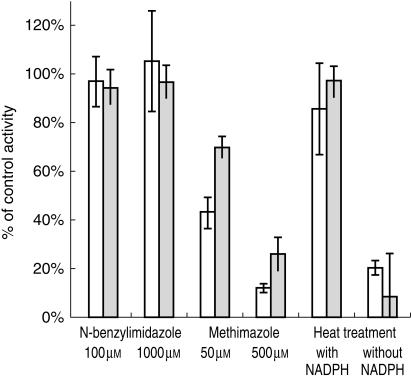
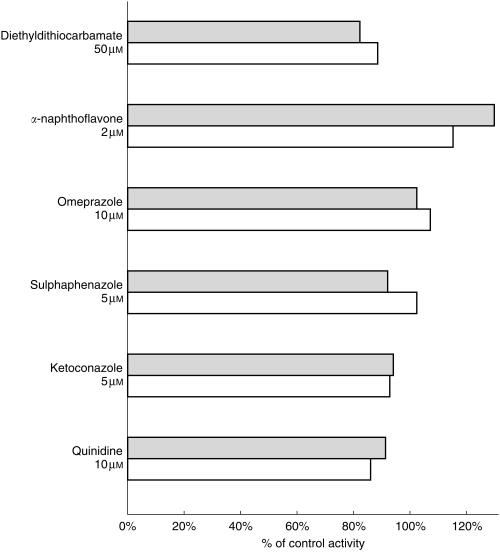
Various experiments were conducted to demonstrate that BZD N-oxidation is an FMO3 mediated reaction and to exclude a contribution of CYP enzymes. Heat treatment of microsomes (5 min, 45 °C) inhibited BZD N-oxidation by more than 90% (mean activity 8.2% of control + 2.3% s.d.). In the presence of NADPH, heat inactivation was prevented (mean activity 97% of control + 18.8% s.d.) (Figure 5). N-benzylimidazole, a nonspecific CYP inhibitor, did not affect BZD-NO formation. Methimazole (500 μm), a competitive inhibitor of FMO activity, decreased BZD-NO formation in a concentration dependent manner by 88% at 10 μm BZD (mean of four livers, s.d. 7.1%) and 74.2% (mean of four livers, s.d. 1.8%) at 250 [AM BZD (Figure 5). Quinidine, ketoconazole, sulphaphenazole, omeprazole, α-naphthoflavone and diethyldithiocarbamate, CYP inhibitors with a higher isoform selectivity than N-benzylimidazole, showed no substantial effect on BZD N-oxidation (Figure 6).
Figure 5.
Effect of heat treatment (45°C, 5 min), methimazole and N-benzylimidazole on BZD-N-oxidation in human liver microsomes. Data represent formation rates relative to control activity (without inhibitor); mean + s.d. of four human livers. Shaded bars: 250 μm BZD, open bars: 10 μm BZD.
Figure 6.
Effect of CYP isoform selective inhibiors on BZD-N-oxidation. Data represent formation rates relative to control activity (without inhibitor) for HL012. Shaded bars: 250 μm BZD, open bars: 10 μm BZD.
To investigate the isoform specificity of BZD N-oxidation, we employed cDNA expressed FMO1, FMO3 and FMO5. Recombinant FMO3 and FMO1 showed comparable BZD-N-oxidase activity while BZD-NO formation by FMO5 was negligible (Table 2). Formation rates were consistent with Michealis-Menten kinetics. Km values were 40.4 μm for FMO3 and 23.6 μm for FMO1 with vmax values of 29.1 and 40.8 nmol mg−1 protein -min−1, respectively. Although the molar concentration of the recombinant FMO was unavailable, the comparison of its BZD-N-oxidase activity with that of human liver can provide an estimate of FMO content. Based on a reported human liver FMO3 content of 100 pmol mg−1 [8], the 4-fold higher Vmax of recombinant FMO3 suggests an FMO3 concentration of approximately 400 pmol mg−1protein in the recombinant enzyme preparation. Corresponding Vmax values for FMO3 and FMO1 are 73 and 100 pmol BZD-NO pmol−1 FMO min−1, respectively. Recombinant CYP 3A4, 2C19, 2D6, 1A2 and 2E1 showed low BZD-N-oxidase activity (< 10 pmol pmol−1 CYP min−1).
Formation rates for N-desmethyl-BZD by human liver microsomes showed vmax values one order of magnitude lower than for BZD N-oxidation and were not influenced by methimazole or heat treatment of microsomes. Formation of N-desmethyl-BZD was catalysed by recombinant CYP 3A4, 2C19 and 2D6 (>9 pmol N-desmethyl-BZD pmol−1 CYP min−1), while CYP 2E1 and 1A2 showed lower formation rates (<5 pmol N-desmethyl-BZD pmol−1 CYP min−1). FMO1, FMO3 and FMO5 did not catalyse the reaction.
Human liver samples from 35 Caucasian subjects were genotyped for six known polymorphisms by PCR-RFLP analysis (Table 1). The mutations causing amino acid changes I66M, P153L and E305X were not found, but the variants M257, G308 and K158 were detected with allele frequencies of 0.114, 0.143 and 0.347, respectively. The E to K amino acid substitution at position 158 significantly altered FMO3 activity in vitro. Subjects homozygous for K158 showed a significant decrease in vmax for benzydamine N-oxidation compared with the subjects carrying at least one wild type allele (EE158+EK158) (P<0.05, Mann-Whitney-U-test) (Table 2). The respective vmax values decreased from a median of 6.9 nmol mg−1 protein min−1 in wild type samples and 6.5 nmol mg−1 protein min−1 in heterozygous individuals to 2.7 nmol mg−1 protein min−1 in individuals homozygous for the mutant allele. Km values were not affected by this mutation (Table 2). No significant changes in enzyme kinetic parameters were evident in samples expressing the M257 or G308 variant of the enzyme compared to the wild type enzyme.
Discussion
As a first step prior to the clinical development of an in vivo test reflecting FMO3 activity, we evaluated the N-oxidation of the antiphlogistic drug benzydamine (Figure 1) as an index reaction for FMO3 activity in human liver microsomes. In previous studies in various animal species, Kawaji et al. [24] demonstrated that benzydamine is N-oxidized by the FMO.
Our results provide evidence that hepatic BZD N-oxidation is almost exclusively catalysed by FMO3 at physiological pH and formation rates in human liver microsomes follow single enzyme Michaelis-Menten kinetics. Heat inactivation of human liver microsomes and use of selective inhibitors for FMO (methimazole) and cytochrome P-450 enzymes (N-benzylimidazole) are commonly used to identify FMO mediated reactions in human liver microsomes [3, 21]. Lack of BZD-NO formation following heat treatment of microsomes and concentration dependent inhibition of BZD N-oxidation by methimazole both indicate a FMO mediated reaction. N-benzylimidazole and various CYP isoform specific inhibitors had no substantial effect on BZD-NO formation at low (10 (μm) or high (250 μm) BZD concentrations. The Km value for recombinant FMO3 (40 μm) falls within the range of Km values determined for human liver microsomes (28–102 μm), as would be expected if BZD-NO formation is mediated by FMO3 in human liver microsomes. Formation rates by recombinant CYP 3A4, 2C19, 2D6, 1A2 and 2E1 were one order of magnitude lower than those determined for recombinant FMO3. Human liver contents of drug metabolizing enzymes have been reported as 100 pmol mg−1 protein for FMO3 [8] and range from 8 pmol mg−1 (CYP2D6) up to 120 pmol mg−1 (CYP3A4) for the various CYP enzymes [25]. Considering the comparable amounts of FMO3 and CYP3A4, the low formation rates for BZD-NO by recombinant CYP confirm the minor contribution of CYP enzymes to BZD N-oxidation indicated by the inhibition studies in human liver microsomes.
To elucidate FMO isoform specificity, we employed cDNA expressed FMO3, FMO5 and FMO 1. While FMO3 and FMO 1 showed high BZD-NO formation rates, activity of FMO5 was negligible (Table 2). Studies on the tissue distribution of FMO indicate that FMO3 and FMO5 are expressed in adult human liver, but FMO3 is the most abundant isoform with FMO3: FMO5 ratios ranging from 2 to 10 [6–8]. FMO1 is expressed in fetal human liver but is confined to kidney in adults [6, 7]. The activity pattern of the FMO isoforms observed in this study is consistent with a previous study using human liver, kidney, intestinal and fetal intestinal microsomes [14]. The role of the kidney in the metabolic clearance of systemically administered drugs is considered to be small and consequently FMO1 is unlikely to contribute substantially to the systemic clearance of BZD in vivo. From these data we conclude that BZD N-oxidation is predominantly, if not exclusively catalysed by FMO3 in humans.
BZD N-demethylation is a second pathway of BZD metabolism (Figure 1) that contibutes less than 10% to overall BZD degradation in human liver microsomes [15, 16]. Our data allowed a qualitative estimate regarding the enzymes involved in this pathway. Recombinant CYP 2D6, 3A4 and 2C19 were the major enzymes catalysing BZD N-demethylation. Since relative liver abundance of CYP 3A4 is about 10-fold higher compared to CYP 2D6 and CYP 2C19, this minor pathway of BZD clearance appears to be mainly mediated by CYP 3A4.
In agreement with previous studies using trimethylamine as a substrate [13, 26], we found a significantly reduced Vmax in samples tested homozygous for the K158 variant compared with those carrying at least one wild type allele (EE158+EK158) (P>0.05, Mann-Whitney-U-test; Kruskal-Wallis-test for all three groups: P = 0.104, Chi square = 4.519, d.f. = 2). Km values for BZD-N-oxidation were not different between the K158 and E158 variant, while an influence of this mutation on Km values has been reported for other substrates [26]. Previous studies with the E. coli expressed M257 variant of FMO3 were inconsistent [13, 26]; an effect of the E308G mutation has not been investigated. In our study, amino acid variants V257M and E308G had no significant effect on enzyme activity, but a larger sample size would be required to exclude any functional significance of these mutations.
FMO generally catalyses N-and S-oxidations of various compounds and both reactions have been used to study kinetic properties of the enzyme [2, 3, 27]. However, it has been shown [1] that sulphur, being a stronger nucleophile than nitrogen, is oxidized at high rates by synthetic, protein-free 4α-hydroperoxyflavin, with the FMO apoenzyme actually accelerating the reaction only about 8000-fold. In contrast, N-oxidations apparently involve a stronger interaction with the protein, leading to a 6 million-fold increase in reaction velocity compared with the rates achieved with hydroperoxyflavin alone. Therefore, N-oxygenated substrates seem more appropriate than S-oxygenated compounds for studies aimed to investigate an impact of alterations in protein structure or expression on enzyme activity.
A number of FMO substrates, such as thiourea and derivatives, methimazole, N,N-dimethylaniline or phe-nothiazine antipsychotics, are useful for in vitro studies but their toxicity or pronounced pharmacological effects preclude them from application to human subjects. Caffeine has recently been proposed as a phenotyping probe for FMO3 [28], but the caffeine concentration used in this in vitro study was 50 mm compared to in vivo concentrations of <50 μm. The relative contribution of FMO3 to caffeine demethylation remains unclear, while other enzymes, namely NAT2, CYP1A2 and CYP2E1, contribute substantially to xanthine metabolism [29, 30]. This might in part explain why independent studies failed to demonstrate a correlation between caffeine metabolic ratios and FMO3 genotype [22, 31].
In contrast, BZD N-oxidation is exclusively mediated by FMO3 in human liver microsomes and an impact of FMO3 genotype on enzyme activity could be demonstrated even in a relatively small number of samples. Apart from its excellent properties as an in vitro index substrate, the nonsteroidal antiphlogistic BZD may be considered a candidate substance for FMO3 phenotyping in vivo. BZD is extensively metabolized, less than 1% of the parent drug is eliminated unchanged in the urine [17]. BZD-NO is the major metabolite in vivo with peak plasma concentrations of approximately 200 ng ml−1 [16].
In the present study we established benzydamine N-oxidation as an index reaction reflecting FMO3 activity in human liver microsomes using an HPLC method with fluorimetric detection. Evidence is provided that FMO3 is the major enzyme mediating BZD N-oxidation in human liver. Homozygous carriers of the K158 variant of the enzyme showed significantly reduced Vmax values compared to those possessing at least one wild type allele. Benzydamine may become an interesting tool to investigate population variability of FMO3 activity in humans, to study drug interactions involving this enzyme, and to assess the contribution of FMO3 to drug metabolism by in vivo clearance correlation analyses.
Acknowledgments
E.S. was the recipient of a grant from the Deutsche Forschungsgemeinschaft, Graduiertenkolleg 426. The authors are grateful to Dr Eigo Takabatake, Setsunan University Osaka, Japan, for generously providing BZD metabolite reference standards.
References
- 1.Poulsen LL, Ziegler DM. Multisubstrate flavin-containing monooxygenases: applications of mechanism to specificity. Chem Biol Interact. 1995;96:57–73. doi: 10.1016/0009-2797(94)03583-t. [DOI] [PubMed] [Google Scholar]
- 2.Ziegler DM. Recent studies on the structure and function of multisubstrate flavin- containing monooxygenases. Annu Rev Pharmacol Toxicol. 1993;33:179–199. doi: 10.1146/annurev.pa.33.040193.001143. [DOI] [PubMed] [Google Scholar]
- 3.Ziegler DM. Flavin-containing monooxygenases: catalytic mechanism and substrate specificities. Drug Metab Rev. 1988;19:1–32. doi: 10.3109/03602538809049617. [DOI] [PubMed] [Google Scholar]
- 4.Cashman JR, Park SB, Berkman CE, Cashman LE. Role of hepatic flavin-containing monooxygenase 3 in drug and chemical metabolism in adult humans. Chem Biol Interact. 1995;96:33–46. doi: 10.1016/0009-2797(94)03581-r. [DOI] [PubMed] [Google Scholar]
- 5.Lawton MP, Cashman JR, Cresteil T, et al. A nomenclature for the mammalian flavin-containing monooxygenase gene family based on amino acid sequence identities. Arch Biochem Biophys. 1994;308:254–257. doi: 10.1006/abbi.1994.1035. [DOI] [PubMed] [Google Scholar]
- 6.Phillips IR, Dolphin CT, Clair P, et al. The molecular biology of the flavin-containing monooxygenases of man. Chem Biol Interact. 1995;96:17–32. doi: 10.1016/0009-2797(94)03580-2. [DOI] [PubMed] [Google Scholar]
- 7.Dolphin CT, Cullingford TE, Shephard EA, Smith RL, Phillips IR. Differential developmental and tissue-specific regulation of expression of the genes encoding three members of the flavin-containing monooxygenase family of man, FMO1, FMO3 and FMO4. EurJBiochem. 1996;235:683–689. doi: 10.1111/j.1432-1033.1996.00683.x. [DOI] [PubMed] [Google Scholar]
- 8.Overby LH, Carver GC, Philpot RM. Quantitation and kinetic properties of hepatic microsomal and recombinant flavin-containing monooxygenases 3 and 5 from humans. Chem Biol Interact. 1997;106:29–45. doi: 10.1016/s0009-2797(97)00055-0. [DOI] [PubMed] [Google Scholar]
- 9.Dolphin CT, Riley JH, Smith RL, Shepard EA, Phillips IA. Structural organisation of the human flavin-containg monooxygenase 3 gene (FMO3), the favored candidate for fish—odor-syndrome, determined directly from genomic DNA. Genomics. 1997;46:260–267. doi: 10.1006/geno.1997.5031. [DOI] [PubMed] [Google Scholar]
- 10.Dolphin CT, Janmohamed A, Smith RL, Shephard EA, Phillips IR. Missense mutation in flavin-containing mono-oxygenase 3 gene, FMO3, underlies fish—odour syndrome. Nat Genet. 1997;17:491–494. doi: 10.1038/ng1297-491. [DOI] [PubMed] [Google Scholar]
- 11.Akerman BR, Forrest S, Chow L, et al. Two novel mutations of the FMO3 gene in a proband with trimethylaminuria. Hum Mutat. 1999;13:376–379. doi: 10.1002/(SICI)1098-1004(1999)13:5<376::AID-HUMU5>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 12.Akerman BR, Lemass H, Chow LM, et al. Trimethylaminuria Is Caused by Mutations of the FMO3 Gene in a North American Cohort. Mol Genet Metab. 1999;68:24–31. doi: 10.1006/mgme.1999.2885. [DOI] [PubMed] [Google Scholar]
- 13.Treacy EP, Akerman BR, Chow LML, et al. Mutations of the flavin-containing monooxygenase gene (FMO3) cause trimethylaminuria, a defect in detoxication. Hum Mol Genet. 1998;7:839–845. doi: 10.1093/hmg/7.5.839. [DOI] [PubMed] [Google Scholar]
- 14.Lang DH, Yeung CK, Peter RM, et al. Isoform specificity of trimethylamine N-oxygenation by human flavin-containing monooxygenase (FMO) and P450 enzymes: selective catalysis by FMO3. Biochem Pharmacol. 1998;56:1005–1012. doi: 10.1016/s0006-2952(98)00218-4. [DOI] [PubMed] [Google Scholar]
- 15.Chasseaud LF, Catanese B. Pharmacokinetics of benzydamine. IntJ Tissue React. 1985;7:195–204. [PubMed] [Google Scholar]
- 16.Catanese B, Lagana A, Marino A, Picollo R, Rotatori M. HPLC determination of benzydamine and its metabolite N-oxide in plasma following oral administration or topical application in man, using fluorimetric detection. Pharmacol Res Commun. 1986;18:385–403. doi: 10.1016/0031-6989(86)90091-3. [DOI] [PubMed] [Google Scholar]
- 17.Baldock GA, Brodie RR, Chasseaud LF, Taylor T. Determination of benzydamine and its N-oxide in biological fluids by high-performance liquid chromatography. J Chromatogr. 1990;529:113–123. doi: 10.1016/s0378-4347(00)83812-8. [DOI] [PubMed] [Google Scholar]
- 18.Kawaji A, Ohara K, Takabatake E. An. assay of flavin-containing monooxygenase activity with benzydamine N- oxidation. Anal Biochem. 1993;214:409–412. doi: 10.1006/abio.1993.1515. [DOI] [PubMed] [Google Scholar]
- 19.Ubeaud G, Schiller KD, Hurbin F, Jaeck D, Coassolo P. Estimation of flavin-containing monooxygenase activity in intact hepatocyte monolayers of rat, hamster, rabbot, dog and human by using N-oxidation of benzydamine. EurJ Pharn Sci. 1999;8:255–260. doi: 10.1016/s0928-0987(99)00016-0. [DOI] [PubMed] [Google Scholar]
- 20.von Moltke LL, Greenblatt DJ, Harmatz JS, Shader RI. Alprazolam metabolism in vitro. Studies of human, monkey, mouse and rat liver microsomes. Pharmacology. 1993;47:268–276. doi: 10.1159/000139107. [DOI] [PubMed] [Google Scholar]
- 21.Grothusen A, Hardt J, Brautigam L, Lang D, Bocker RA. convenient method to discriminate between cytochrome P450 enzymes and flavin-containing monooxygenases in human liver microsomes. Arch Toxicol. 1996;71:64–71. doi: 10.1007/s002040050359. [DOI] [PubMed] [Google Scholar]
- 22.Sachse C, Ruschen S, Dettling M, et al. Flavin monooxygenase 3 (FMO3) polymorphism in a Caucasian population: allele frequencies, mutation linkage and functional effects on clozapine and caffeine metabolism. Clin Pharmacol Ther. 1999;66:431–438. doi: 10.1053/cp.1999.v66.a102203. [DOI] [PubMed] [Google Scholar]
- 23.Segel IH. Enzyme kinetics: Behavior and analysis of rapid equilibrium and steady state systems. New York: Wiley ↦ Sons, Inc.; 1975. [Google Scholar]
- 24.Kawaji A, Isobe M, Takabatake E. Differences in enzymatic properties of flavin-containing monooxygenase in brain microsomes of rat, mouse, hamster, guinea pig and rabbit. Biol Pharm Bull. 1997;20:917–919. doi: 10.1248/bpb.20.917. [DOI] [PubMed] [Google Scholar]
- 25.Shimada T, Yamazaki H, Mimura M, Inui Y, Guengerich FP. Interindividual variations in human liver cytochrome P-450 enzymes involved in the oxidation of drugs, carcinogens and toxic chemicals: Studies with liver microsomes of 30 Japanese and 30 Caucasians. J Pharmacol Exp Ther. 1994;270:414–423. [PubMed] [Google Scholar]
- 26.Cashman JR, Akerman BR, Forrest SM, Treacy EP. Population-specific polymorphisms of the human FMO3 gene: significance for detoxication. Drug Metab Dispos. 2000;28:169–173. [PubMed] [Google Scholar]
- 27.Ziegler DM. Flavin-containing monooxygenases: enzymes adapted for multisubstrate specificity. Trends Pharmacol Sci. 1990;11:321–324. doi: 10.1016/0165-6147(90)90235-z. [DOI] [PubMed] [Google Scholar]
- 28.Chung WG, Cha YN. Oxidation of caffeine to theobromine and theophylline is catalyzed primarily by flavin-containing monooxygenase in liver microsomes. Biochem Biophys Res Commun. 1997;235:685–688. doi: 10.1006/bbrc.1997.6866. [DOI] [PubMed] [Google Scholar]
- 29.Fuhr U, Rost KL, Engelhardt R, et al. Evaluation of caffeine as a test drug for CYP1A2, NAT2 and CYP2E1 in man by in vivo versus in vitro correlations. Pharmacogenetics. 1996;6:159–176. doi: 10.1097/00008571-199604000-00003. [DOI] [PubMed] [Google Scholar]
- 30.Rostami-Hodjegan A, Nurminen S, Jackson PR, Tucker GT. Caffeine urinary metabolite ratios as markers of enzyme activity, a theoretical assessment. Pharmacogenetics. 1996;6:121–149. doi: 10.1097/00008571-199604000-00001. [DOI] [PubMed] [Google Scholar]
- 31.Park CS, Chung WG, Kang JH, et al. Phenotyping of flavin-containing monooxygenase using caffeine metabolism and genotyping of FMO3 gene in a Korean population. Pharmacogenetics. 1999;9:155–164. [PubMed] [Google Scholar]