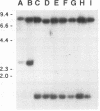
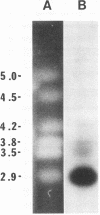
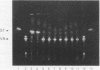
Abstract
Aspergillus nidulans produces the carcinogenic mycotoxin sterigmatocystin (ST), the next-to-last precursor in the aflatoxin (AF) biosynthetic pathway found in the closely related fungi Aspergillus flavus and Aspergillus parasiticus. We identified and characterized an A. nidulans gene, verA, that is required for converting the AF precursor versicolorin A to ST. verA is closely related to several polyketide biosynthetic genes involved in polyketide production in Streptomyces spp. and exhibits extended sequence similarity to A. parasiticus ver-1, a gene proposed to encode an enzyme involved in converting versicolorin A to ST. By performing a sequence analysis of the region 3' to verA, we identified two additional open reading frames, designated ORF1 and ORF2. ORF2 is closely related to a number of cytochrome P-450 monooxygenases, while ORF1 shares identity with the gamma subunit of translation elongation factor 1. Given that several steps in the ST-AF pathway may require monooxygenase activity and that AF biosynthetic genes are clustered in A. flavus and A. parasiticus, we suggest that verA may be part of a cluster of genes required for ST biosynthesis. We disrupted the verA coding region by inserting the A. nidulans argB gene into the center of the coding region and transformed an A. nidulans argB2 mutant to arginine prototrophy. Seven transformants that produced DNA patterns indicative of a verA disruption event were grown under ST-inducing conditions, and all of the transformants produced versicolorin A but negligible amounts of ST (200-fold to almost 1,000-fold less than the wild type), confirming the hypothesis that verA encodes an enzyme necessary for converting versicolorin A to ST.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adamson R. H. Induction of hepatocellular carcinoma in nonhuman primates by chemical carcinogens. Cancer Detect Prev. 1989;14(2):215–219. [PubMed] [Google Scholar]
- Baertschi S. W., Raney K. D., Shimada T., Harris T. M., Guengerich F. P. Comparison of rates of enzymatic oxidation of aflatoxin B1, aflatoxin G1, and sterigmatocystin and activities of the epoxides in forming guanyl-N7 adducts and inducing different genetic responses. Chem Res Toxicol. 1989 Mar-Apr;2(2):114–112. doi: 10.1021/tx00008a008. [DOI] [PubMed] [Google Scholar]
- Brody H., Carbon J. Electrophoretic karyotype of Aspergillus nidulans. Proc Natl Acad Sci U S A. 1989 Aug;86(16):6260–6263. doi: 10.1073/pnas.86.16.6260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody H., Griffith J., Cuticchia A. J., Arnold J., Timberlake W. E. Chromosome-specific recombinant DNA libraries from the fungus Aspergillus nidulans. Nucleic Acids Res. 1991 Jun 11;19(11):3105–3109. doi: 10.1093/nar/19.11.3105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullerman L. B., Hartman P. A., Ayres J. C. Aflatoxin production in meats. II. Aged dry salamis and aged country cured hams. Appl Microbiol. 1969 Nov;18(5):718–722. doi: 10.1128/am.18.5.718-722.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrez D., Janssens W., Degrave P., van den Hondel C. A., Kinghorn J. R., Fiers W., Contreras R. Heterologous gene expression by filamentous fungi: secretion of human interleukin-6 by Aspergillus nidulans. Gene. 1990 Oct 15;94(2):147–154. doi: 10.1016/0378-1119(90)90381-z. [DOI] [PubMed] [Google Scholar]
- Chang P. K., Cary J. W., Bhatnagar D., Cleveland T. E., Bennett J. W., Linz J. E., Woloshuk C. P., Payne G. A. Cloning of the Aspergillus parasiticus apa-2 gene associated with the regulation of aflatoxin biosynthesis. Appl Environ Microbiol. 1993 Oct;59(10):3273–3279. doi: 10.1128/aem.59.10.3273-3279.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang P. K., Skory C. D., Linz J. E. Cloning of a gene associated with aflatoxin B1 biosynthesis in Aspergillus parasiticus. Curr Genet. 1992 Mar;21(3):231–233. doi: 10.1007/BF00336846. [DOI] [PubMed] [Google Scholar]
- Denison S. H., Käfer E., May G. S. Mutation in the bimD gene of Aspergillus nidulans confers a conditional mitotic block and sensitivity to DNA damaging agents. Genetics. 1993 Aug;134(4):1085–1096. doi: 10.1093/genetics/134.4.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis O. J., Jr, Ware G. M., Carman A. S., Kirschenheuter G. P., Kuan S. S., Newell R. F. Thin-layer chromatographic determination of sterigmatocystin in cheese: interlaboratory study. J Assoc Off Anal Chem. 1987 Sep-Oct;70(5):842–844. [PubMed] [Google Scholar]
- Fujii K., Kurata H., Odashima S., Hatsuda Y. Tumor induction by a single subcutaneous injection of sterigmatocystin in newborn mice. Cancer Res. 1976 May;36(5):1615–1618. [PubMed] [Google Scholar]
- Hohn T. M., McCormick S. P., Desjardins A. E. Evidence for a gene cluster involving trichothecene-pathway biosynthetic genes in Fusarium sporotrichioides. Curr Genet. 1993 Oct;24(4):291–295. doi: 10.1007/BF00336778. [DOI] [PubMed] [Google Scholar]
- Holzapfel C. W., Purchase I. F., Steyn P. S., Gouws L. The toxicity and chemical assay of sterigmatocystin, a carcinogenic mycotoxin, and its isolation from two new fungal sources. S Afr Med J. 1966 Dec 17;40(45):1100–1101. [PubMed] [Google Scholar]
- Hopwood D. A., Sherman D. H. Molecular genetics of polyketides and its comparison to fatty acid biosynthesis. Annu Rev Genet. 1990;24:37–66. doi: 10.1146/annurev.ge.24.120190.000345. [DOI] [PubMed] [Google Scholar]
- Jelinek C. F., Pohland A. E., Wood G. E. Worldwide occurrence of mycotoxins in foods and feeds--an update. J Assoc Off Anal Chem. 1989 Mar-Apr;72(2):223–230. [PubMed] [Google Scholar]
- Keller N. P., Dischinger H. C., Jr, Bhatnagar D., Cleveland T. E., Ullah A. H. Purification of a 40-kilodalton methyltransferase active in the aflatoxin biosynthetic pathway. Appl Environ Microbiol. 1993 Feb;59(2):479–484. doi: 10.1128/aem.59.2.479-484.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafont P., Siriwardana M. G., DeBoer E. Contamination of dairy products by fungal metabolites. J Environ Pathol Toxicol Oncol. 1990 May-Jun;10(3):99–102. [PubMed] [Google Scholar]
- Maessen G. D., Amons R., Zeelen J. P., Möller W. Primary structure of elongation factor 1 gamma from Artemia. FEBS Lett. 1987 Oct 19;223(1):181–186. doi: 10.1016/0014-5793(87)80532-x. [DOI] [PubMed] [Google Scholar]
- Miller B. L., Miller K. Y., Timberlake W. E. Direct and indirect gene replacements in Aspergillus nidulans. Mol Cell Biol. 1985 Jul;5(7):1714–1721. doi: 10.1128/mcb.5.7.1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northolt M. D., van Egmond H. P., Soentoro P., Deijll E. Fungal growth and the presence of sterigmatocystin in hard cheese. J Assoc Off Anal Chem. 1980 Jan;63(1):115–119. [PubMed] [Google Scholar]
- Payne G. A., Nystrom G. J., Bhatnagar D., Cleveland T. E., Woloshuk C. P. Cloning of the afl-2 gene involved in aflatoxin biosynthesis from Aspergillus flavus. Appl Environ Microbiol. 1993 Jan;59(1):156–162. doi: 10.1128/aem.59.1.156-162.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott P. M., Van Walbeek W., Kennedy B., Anyeti D. Mycotoxins (ochratoxin A, citrinin, and sterigmatocystin) and toxigenic fungi in grains and other agricultural products. J Agric Food Chem. 1972 Nov-Dec;20(6):1103–1109. doi: 10.1021/jf60184a010. [DOI] [PubMed] [Google Scholar]
- Shimada T., Guengerich F. P. Evidence for cytochrome P-450NF, the nifedipine oxidase, being the principal enzyme involved in the bioactivation of aflatoxins in human liver. Proc Natl Acad Sci U S A. 1989 Jan;86(2):462–465. doi: 10.1073/pnas.86.2.462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skory C. D., Chang P. K., Cary J., Linz J. E. Isolation and characterization of a gene from Aspergillus parasiticus associated with the conversion of versicolorin A to sterigmatocystin in aflatoxin biosynthesis. Appl Environ Microbiol. 1992 Nov;58(11):3527–3537. doi: 10.1128/aem.58.11.3527-3537.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skory C. D., Chang P. K., Linz J. E. Regulated expression of the nor-1 and ver-1 genes associated with aflatoxin biosynthesis. Appl Environ Microbiol. 1993 May;59(5):1642–1646. doi: 10.1128/aem.59.5.1642-1646.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D. J., Burnham M. K., Bull J. H., Hodgson J. E., Ward J. M., Browne P., Brown J., Barton B., Earl A. J., Turner G. Beta-lactam antibiotic biosynthetic genes have been conserved in clusters in prokaryotes and eukaryotes. EMBO J. 1990 Mar;9(3):741–747. doi: 10.1002/j.1460-2075.1990.tb08168.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srám R. J., Binková B., Dobiás L., Rössner P., Topinka J., Veselá D., Veselý D., Stejskalová J., Bavorová H., Rericha V. Monitoring genotoxic exposure in uranium miners. Environ Health Perspect. 1993 Mar;99:303–305. doi: 10.1289/ehp.99-1567033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stack M., Rodricks J. V. Method for analysis and chemical confirmation of sterigmatocystin. J Assoc Off Anal Chem. 1971 Jan;54(1):86–90. [PubMed] [Google Scholar]
- Wu T. S., Linz J. E. Recombinational inactivation of the gene encoding nitrate reductase in Aspergillus parasiticus. Appl Environ Microbiol. 1993 Sep;59(9):2998–3002. doi: 10.1128/aem.59.9.2998-3002.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yabe K., Hamasaki T. Stereochemistry during aflatoxin biosynthesis: cyclase reaction in the conversion of versiconal to versicolorin B and racemization of versiconal hemiacetal acetate. Appl Environ Microbiol. 1993 Aug;59(8):2493–2500. doi: 10.1128/aem.59.8.2493-2500.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokotani N., Bernhardt R., Sogawa K., Kusunose E., Gotoh O., Kusunose M., Fujii-Kuriyama Y. Two forms of omega-hydroxylase toward prostaglandin A and laurate. cDNA cloning and their expression. J Biol Chem. 1989 Dec 25;264(36):21665–21669. [PubMed] [Google Scholar]
- Yu J., Cary J. W., Bhatnagar D., Cleveland T. E., Keller N. P., Chu F. S. Cloning and characterization of a cDNA from Aspergillus parasiticus encoding an O-methyltransferase involved in aflatoxin biosynthesis. Appl Environ Microbiol. 1993 Nov;59(11):3564–3571. doi: 10.1128/aem.59.11.3564-3571.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]