Abstract
Capillaries play a critical role in cardiovascular function as the point of exchange of nutrients and waste products between the tissues and circulation. Studies of capillary function in man are limited by access to the vascular bed. However, skin capillaries can readily be studied by the technique of capillaroscopy which enables the investigator to assess morphology, density and blood flow velocity. It is also possible to estimate capillary pressure by direct cannulation using glass micropipettes. This review will describe the techniques used to make these assessments and will outline some of the changes that are seen in health and disease.
Keywords: capillary blood velocity, capillary density, skin microcirculation
Introduction
Capillaries play a vital role in the exchange of nutrients and waste products (the primary function of the cardiovascular system). The density of capillaries determines the total surface area available for exchange, the maximum distance between a cell and blood and thus diffusion time, and contributes to the total resistance of the capillary bed. Alterations in capillary blood flow and pressure may influence the transcapillary exchange processes, the magnitude of this effect being influenced by the lipid-solubility and size of the molecules concerned, and the tissue metabolic requirements. Capillaries also play an important role in tissue fluid homeostasis. The transport of fluid across the microvascular wall is controlled by Starling's forces the most variable of which is capillary pressure with the effect that small changes in capillary pressure can have marked effects on transcapillary fluid flux [1]. The regulation of capillary flow, pressure or the movement of molecules across the capillary wall has been discussed in reviews elsewhere [1, 2]
Examination of human capillaries is limited by access to this vascular bed and the techniques available. Although one might imagine that blood flow, pressure and the mechanisms controlling blood flow at the capillary level can be deduced by extrapolating from the findings in larger vessels, the information from animal studies suggests that this is not the case [3–5]. Thus there is no alternative but to investigate capillary function directly. In man investigations are confined to capillaries of the retina [6], lip [7] and skin [8]. Most work has been carried out on skin capillaries which will therefore be the main focus of this review. Before describing the methods used to examine human skin capillaries, skin vascular anatomy will be described briefly.
The anatomy of the cutaneous microvasculature
The skin blood supply originates from perforating vessels rising from the underlying muscles and subcutaneous fat to form a plexus, the lower horizontal plexus, at the dermal–subcutaneous interface. From the lower plexus, paired arterioles and venules rise to form direct connections with a second plexus, the subpapillary plexus which is situated in the papillary dermis, and from this the capillary loops of the dermal papillae arise. The majority of the microvasculature of the skin resides in the papillary dermis 1–2 mm below the surface of the skin. Microvessels in the papillary dermis range in size from 10 to 35 μm whereas those in the mid to deep dermis are 40–50 μm with an occasional arteriole as large as 100 μm being observed [9].
Changes in microvascular anatomy with site and age
Microvascular anatomy, particularly the capillary loops, may vary according to the skin area examined and the age of the subject. Uniquely in the toe and finger nail fold, the terminal row of dermal capillary loops lie parallel to the surface of the skin. Moving proximally along the digit the orientation of capillaries changes to become perpendicular or oblique to the surface. Both orientations of capillaries are also found at other skin sites although the relative numbers vary. The development of the capillary loop can also vary, for example in forearm skin where the dermal papillae are not well developed the arterioles connect to capillaries which course close to the dermal–epidermal interface before joining a post capillary venule of the subpapillary plexus. Total capillary density varies according to the area of skin examined and differences over small areas such as the dorsum of the foot have been reported [10]. Ageing is accompanied by a loss in dermal volume, a reduction in capillary density [11], shortened capillary loops, and rarefaction of larger microvessels [12]
Examination of human capillaries-a historical perspective
Thirty years after William Harvey elegantly first described the blood circulation, the tiny vessels which link the arterial and venous tree were identified [13]. Visualization of blood flow in these vessels, which are similar in diameter to red blood cells, was first made in frog capillaries by Anthony van Leeuwenhoeck and in 1879 the first microscopic examinations of human skin capillaries were conducted [14]. In 1922 Müller [15] published a book in which his examinations of skin capillaries using microscopy were illustrated in colour by artists, and show the movement of red blood cells in human nailfold capillaries and the morphology of capillaries in several disease states.
The initial measurements of capillary blood velocity were made in 1919 by Basher [16]. Subsequent measurements were reported in 1964 by Zimmer and Demis who used a microscope-television system to study the flow dynamics in human skin capillaries [17]. A new television-microscopy system introduced in 1974 by Bollinger and colleagues [18] showed that it was possible, using a frame-to-frame analysis of the movement of plasma gaps along a capillary, to examine capillary dynamics in healthy controls and patients. This was improved and simplified by Fagrell et al.[8] who used a video-photometric cross correlation technique which allowed continuous assessment of capillary velocity over longer periods of time and could, although technically difficult, be used by experienced personnel in the clinic (see later for details).
Cannulation of human capillaries and direct measurement of capillary pressure were first performed by Carrier and Rehberg in 1923 [19]. Even before this time indirect methods to estimate capillary pressure were reported [20] although the values obtained do not agree with direct measurements. In 1930 Landis [21] published his seminal paper on measurement of capillary and venous pressure and the effects of physiological and pharmacological interventions. His technique involved introducing a micropipette attached to a water manometer into a capillary and adjusting manometric pressure until blood did not enter the micropipette tip. Manometric pressure at this equilibrium point represented mean capillary pressure. Further early studies using the manometric system in patients with hypertension [22], heart failure [23], and glomerulonephritis [24] were published and then interest in capillary pressure dwindled until the late 1970s. The disadvantage of the manometric technique is that only averaged pressure can be determined. The first dynamic measurements of human capillary pressure were described in 1979 using a servo-nulling system similar to that used today [25].
Current investigation of human skin capillaries
Capillary microscopy or capillaroscopy allows visualization of a living system in real time, it may be referred to as intravital capillary microscopy or intravital capillaroscopy although in the clinical setting ‘intravital’ is often omitted. Capillaroscopy provides a 2-D projection of a 3-D network of capillaries. In combination with television and video and/or computer technology capillaroscopy generates high contrast images of skin capillaries on videotape, computer disc or photograph.
The native technique may be used to assess capillary morphology, capillary density, capillary blood velocity (dynamic capillaroscopy), capillary red cell column width or to facilitate direct capillary cannulation. In combination with intravenous administration of fluorescent dyes, e.g. sodium fluorescein or indocyanide green (fluorescence video microscopy or fluorescence angiography), capillaroscopy can be used to examine heterogeneity of capillary flow distribution, to visualize structures not detectable by native capillaroscopy such as capillary aneurysms, and to follow the transcapillary diffusion of tracer as a marker of capillary permeability to small solutes [26]. The skin pigment, melanin, absorbs light strongly in the visible spectrum making capillaroscopy difficult in highly pigmented skin. However fluorescence video microscopy can be used to estimate capillary density in these individuals. Furthermore the intravital microscopy technique has been used in combination with subepidermal injections of FITC dextran to examine microlymphatic network function and microlymphatic capillary pressure (using the servonulling system) in human skin [27, 28].
Methods
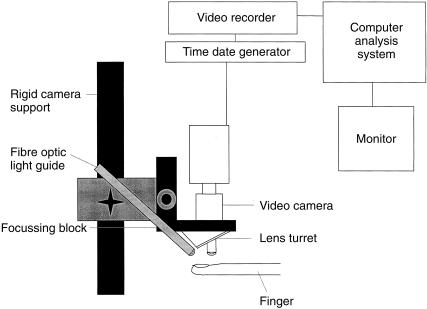
Equipment requirements for a capillary microscope Figure 1
Figure 1.
A diagrammatic representation of a capillary microscope.
Clinical examination is facilitated if the microscope can be moved over the skin area of interest rather than moving the patient. To achieve this the optics, on a standard mounting block, can be attached to a focusing block which is itself attached to an arm to allow movement up and down, side to side and preferably tilting in two planes. A very light sensitive black and white camera (e.g. Philips CCD camera LDH 0703/30) is mounted directly over the lens system and the skin is illuminated by a 50 or 100 W mercury vapor light (Zeiss) directed to the tissue either through the lens (epi-illumination) or via a fibre optic light guide (Figure 1). The lens should have long working distances (1–1.5 cm) and preferably an adjustable diaphragm to control light input to the camera. The microscope objective should be at right angles to the skin surface. Illumination is one of the most important parts of the capillary microscope. The emission spectrum of mercury vapour is similar to the absorption spectrum of haemoglobin (370–450 nm), thus red blood cells appear black in the image and it is the movement of these cells which is observed when examining capillaries under the microscope. A heat filter and other filters, such as blue or green, are also required to enhance the contrast sufficient for analysis. The particular filter requirements will depend upon: the nature of the illumination, the spectral characteristics of the Mercury vapour light (which alter with hours of usage), and the patient's skin characteristics. The images are displayed on a high resolution black and white monitor (e.g. Hitachi VM-AE906/K) with final magnification 120–400 fold and stored on sVHS video tapes, computer discs or as photographs. Time and date marks on the images allow subsequent identification of a study. Verbal annotation of video tapes may be useful. Further details of equipment requirements are published elsewhere see reviews [26, 29]. A complete, freely moving commercial capillary microscope is not available however, CapiFlow (Stockholm, Sweden) and Leica (UK) market modified standard microscopes in an attempt to fill the need. Specifications for cameras, and to a lesser extent monitors and lens, change so rapidly that it is always necessary to reassess a variety of combinations whenever a new system is purchased.
Experimental conditions for the examination of human capillaries
Due to the thermoregulatory properties of skin and the susceptibility of capillary blood flow and pressure to alterations in venous pressure, sympathetic or sudomotor drive, etc., representative and valid readings for capillary density, red blood cell velocity and pressure will only be obtained if subjects are studied under carefully controlled conditions. Thus the position in which subjects are examined should be standardized (supine or seated, hand at heart level) and factors such as food intake, smoking, time of day, menstrual cycle, should be carefully controlled. Studies should take place in an undisturbed quiet room with a defined stable temperature, after a period of acclimatization which lasts until skin temperature has stabilized. Skin temperature should be recorded as a variable which influences skin blood flow.
A factor common to all examinations using capillaroscopy is the requirement for excellent images, in particular high black/white contrast with no movement artifacts. To achieve this it is necessary to immobilize the digit but this must be achieved without affecting blood flow. Blood flow can be artifactually altered by increasing venous pressure, by pressing on the nail, by occluding arterial inflow, etc. Stationary pictures may be obtained by asking the subject to relax when lying comfortably with the arm and palm of the hand at heart level supported on a padded table alongside the body, the elbow slightly bent. The finger can be supported in a holder moulded to fit. A finger stabiliser attached to the microscope lens may be necessary if long dynamic assessments are being made.
For all applications of capillaroscopy a good image will only be obtained when the considerable scattering of light at the air/stratum corneum interface is reduced. This can be achieved by applying a thin layer of paraffin oil, glycerine or clear nail varnish to the skin surface. Before the capillaries are examined, time should be allowed for the microcirculation to recover from the perturbation caused by the application of these agents.
The assessment of capillary morphology
Morphological examination of capillaries can be performed by viewing the skin at low power (5–100x) through a simple light microscope or an opthalmoscope. Many but not all morphological assessments have been performed at the finger nailfold. Capillary morphology may be altered by local trauma to the nailfold as may occur in nail biting, or in those engaged in manual work such as builders or farmers. It is therefore important to note such factors on any assessment forms. When assessments are being made at other skin sites it is important to document the clinical nature of the area since morphology can differ markedly in adjacent areas, e.g. avascular atrophie blanche areas compared to adjacent areas of enlarged glomerular like capillaries in chronic venous insufficiency [30].
The assessment of capillary density
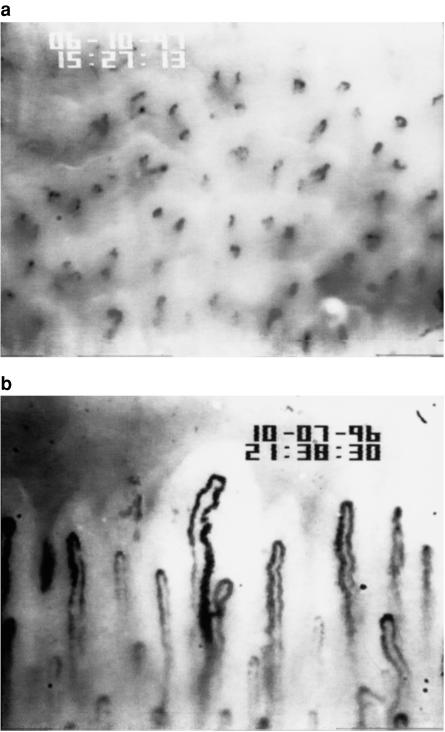
Capillary density is the number of capillaries per unit area of skin. Essentially it is measured by recording images from the capillary microscope and then counting the capillaries in a known area of skin. Depending on the skin area under investigation the capillaries will appear as black dots, if the capillaries are perpendicular to the surface, (Figure 2a), or as lines if the capillaries are lying obliquely, or a mixture of the two. It should be noted that the capillary wall cannot be seen and therefore if a capillary is not perfused with red blood cells it will not be visualized under resting conditions using native capillaroscopy.
Figure 2.
a) Skin capillaries on the dorsum of the finger. Magnification 173 fold. b) Normal nailfold capillaries. Magnification 173 fold
The exact procedure differs from laboratory to laboratory. Four black and white photographs may, for example, be taken around a central point tattooed on the skin and capillaries counted from the photographs. Alternatively six sequential microscopy fields may be recorded for 2 min each (whilst the focusing level is systematically adjusted) and the capillaries counted whilst playing back the video. Ideally counting should be performed whilst observing the movement of red blood cells and plasma gaps through the capillaries during video replay since this may clarify whether a given black dot is a capillary, whether a knot of vessels is one capillary or two and it will allow the identification of all vessels perfused in the 2 min period rather than those perfused at the instant of a photograph. It is also possible to combine the two methods described above by counting capillaries from video prints as well as still frame photographs.
Recordings of capillary density can estimate the functional density of capillaries under a given condition or the total density of vessels. In the latter case those not perfused at rest must be filled with blood in order to be visualized. This may be achieved either by increasing venous pressure by 40 or 60 mmHg for a few minutes (10 [31] or 2, respectively [32]) using a sphygomanometer cuff or by using an arterial occlusion and counting capillaires during the hyperaemia following cuff release [33]. The disadvantage of the latter is that, in health, the hyperaemia is transient (< 10 s), it is therefore difficult to record the response (subjects move on cuff release, the image needs to be focused at different depths to ensure all the capillaries at different planes have been visualized, etc.). Furthermore the hyperaemia is often followed by a transitory reduction in flow to below resting values which makes it even more difficult to record vessels. The increase in capillary density is around 12–18% in health. Fluorescence angiography may also be used to identify capillaries which are perfused with plasma only and are therefore not visible under white light.
Antonios et al. have just performed a direct comparison between arterial and venous occlusion methods of obtaining maximal capillary density and finds venous occlusion to be superior [34]. It is uncertain how much of the increase in capillary density following intervention is due to improved visualization of the capillaires, which appear more black on the image due to the increased number of red cells, and how much is truly due to recruitment of previously unperfused capillaries. It is for this reason that the utmost care must be taken to ensure that all flowing capillaries are recorded during the baseline measurements. Reproducibility of the technique for resting capillary density is 5–8% and for capillary density following intervention is 7% [31, 32].
The use of capillaroscopy in highly pigmented skin is difficult. Native capillaroscopy is only possible in the lighter pigmented areas and these may not be representative of all skin areas. Capillary density can, however, be determined in pigmented skin using fluorescence angiography.
In recent years in addition to determining the numbers of capillaries per unit area of skin further analysis of vessel anatomy have been undertaken. Stereological point counting methods have, for example, been used to assess the vessel volume fraction of skin. This is derived from the Delesse principle that the volume fraction of a component of a three-dimensional solid can be estimated from the area fraction of that component in a two-dimensional state [35]. Such techniques are increasing in popularity but are not as yet widely used in this field.
Assessments of capillary blood velocity (CBV)
The movement of red blood cells around a capillary is easily visualized under the microscope (Figure 2b). The capillary wall cannot be visualized and fluorescent dye studies using indocyanide green have demonstrated that the red cell column width occupies only 67%–68% of the total capillary diameter [36]. True capillary blood flow (mls min−1) therefore cannot be quantified by native capillaroscopic techniques. Capillary red blood cell velocity and an estimate of capillary blood flow (derived using red cell column width as an estimate of capillary diameter) can be obtained but, using the latter to examine the effect of interventions or differences between groups of subjects assumes that the red cell column width remains a similar proportion of the total capillary diameter.
Differences in depth or velocity of blood flow in capillaries makes it easier to see blood flow in some capillaries. CBV also fluctuates in any given capillary. Without care it is easy to subconsciously select the slower capillaries which are most eye catching and are easiest to analyse. To prevent this bias a systematic examination should be undertaken in which recordings start at one side of the nailfold and sequentially record all capillaries until the required number is reached. Each recording must be long enough to take into account temporal variation in flow, in normal individuals a 2 min recording should be adequate but in some diseased states a longer period may be necessary. A manual estimate of the total time that blood has stopped flowing should be recorded since zero velocity cannot be recorded using the automated systems and to exclude these data would artifically raise the mean CBV for an individual.
As well as measuring resting capillary blood velocity the response (usually time to peak or trough, peak or nadir CBV during intervention, time to recover resting flow) to various interventions can be investigated. This approach may minimize differences between capillaries, standardize the temporal changes in flow between vessels and thus reduce variability between individuals. Arterial or venous occlusion should be performed by inflating a tiny cuff around the base of the finger rather than the arm [37] as the former maximizes the rate of increase in pressure and improves reproducibility. Responses to local cooling [38, 39], can be investigated as can the effects of the local application of agents by iontophoresis [40]. Each of these procedures requires the assessment of reproducibility by any potential users since considerable variability in responses will occur unless great care is taken. When used appropriately reproducibility is of the order of 12% for the reduction of CBV seen during local cooling [41, 42], 18–25% for the hyperaemic peak red blood cell velocity and 19% for the time to peak velocity following 1 min arterial occlusion [43, 44].
Several methods are available to determine CBV. The traditional methods, of frame-to-frame analysis and the flying spot technique [45] have been superceded by automated measurements of CBV using a cross correlation technique [46, 47]. Although originally analogue procedures, commercially available computerized techniques are now available [48, 49]. In spatial correlation mode two photometric windows are generated on the video image, placed over a straight section of the capillary limb and the intensities of the images in the two windows, which fluctuate due to the passage of red blood cells, plasma gaps or white cells, are monitored over time. The computer uses cross correlation techniques to calculate CBV. The principle of this techique is the determination of the time interval by which the upstream window has to be delayed to achieve maximum correlation with the signal from the downstream window, knowing the distance between the two windows, the computer can thereby calculate velocity. Although in principle the technique is easy to use, in practise it requires excellent static images and a considerable amount of user experience to place windows of the correct size, the correct distance apart in order to achieve adequate correlations. It does not measure zero velocity unless being used in temporal mode when the measurement line is defined along the length of the capillary. The analysis remains very time consuming especially as it is necessary to measure the velocity in several capillaries per subject to obtain a representative value. Its advantages are that it can measure fluctations in red blood cell velocity from moment to moment. Several other novel methods are also being evaluated for the measurement of CBV such as the spatial shift alignment method [50].
To calculate red blood cell flow, measurement of red blood cell column width is required. In the past this was measured manually using calipers however, computerized analysis using a technique which involves shearing of the vessel image along a line perpendicular to the vessel is now more often used. Once the vessel has been sheared one of the images is moved until the near edge of its red cell column is aligned with the far edge of the red cell column in the unmoved image. The distance the image has to move to achieve this is equal to the red cell column width.
The assessment of capillary perfusion using the capillary anemometer
This new application of laser Doppler anemometry to measure perfusion in a single capillary offers the opportunity to measure capillary flow at a site other than the nailfold. It involves directing a focused laser beam on to a limb of a single capillary and using Doppler principles to monitor capillary perfusion. It is able to measure greater velocities than can be detected using standard camera and video techniques and clear cardiac pulsatility of capillary flow can be detected especially in high flow conditions. Few data are so far available with the technique although the traditional relationship of capillary perfusion and skin temperature has been confirmed, and marked increases in flow following the application of acetylcholine and abnormalities of capillary perfusion in patients with diabetes have also been described [40, 51, 52].
The assessment of capillary pressure
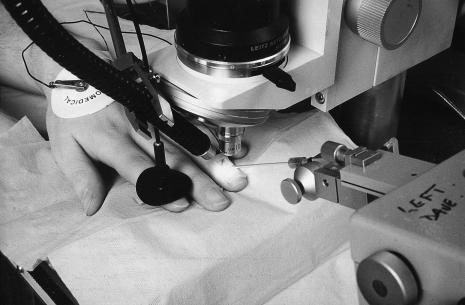
Nailfold capillaries can be directly cannulated using glass micropipettes (tip diameters of 5–10 μm) held in a micromanipulator at an angle of approximately 50° to the skin surface (Figure 3). It is usually necessary to remove the dead cornified skin layer to facilitate cannulation. Cannulations at the apex of the capillary loop allow accurate pipette positioning with minimal perturbation to blood flow and thus pressure. Pressure is measured using a resistance null-balance feedback system (IPM Inc., San Diego, California) originally described by Wiederheilm and Intaglietta [53, 54]. Mean capillary pressure and pre and post cannulation zero values are calculated. An average waveform is obtained by using the R-wave of the ECG to superimpose 10–24 capillary pressure waveforms and the capillary pulse pressure amplitude (CPPA), the time from the R-wave to the foot of the systolic upstroke (systolic arrival time) and the peak pressure are obtained. Fast Fourier transformation may be applied to examine the high frequency components of the waveform.
Figure 3.
Measurement of nailfold capillary pressure.
For each subject capillary pressure is defined as the average pressure obtained from a minimum of three capillaries each cannulated at the apex of the capillary loop. In contrast to capillary red blood cell velocity, capillary pressure is a remarkably stable parameter. Reproducibility of pressure measured in nine capillaries across the nailfold at the same visit is 5.4 ± 2.0% in health and in six subjects studied on three separate occasion the mean coefficient of variation was 5.2 ± 3.6% [55].
Capillaroscopy and capillary pressure in health
The effects of age
The reductions in dermal volume which accompany ageing improve visualization of vessels in the subpapillary plexus. There is a striking reduction in capillary density with age and capillary loops are shortened [11, 12].
Capillary pressure physiology
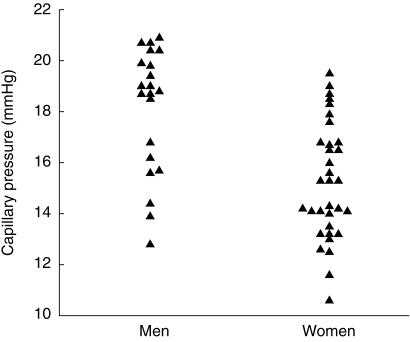
Normal capillary pressure, measured at the apex of the capillary loop with the capillary at heart level, ranges from 10.5 to 22.5 mmHg (Figure 4). It is lower in premenopausal women than in postmenopausal women or in men and does not correlate with brachial artery blood pressure. Cardiac pulsations are clearly transmitted to the capillary, pulse pressures as low as 0.5 mmHg and as high as 12 mmHg have been recorded under resting conditions in health [55]. Capillary pressure increases in response to an increase in venous pressure [56, 57], but appears to be protected from acute alterations in arterial pressure [58]. During local cooling capillary pressure changes little but in the post cooling period CBV, capillary pressure and capillary pulse pressure amplitude increase in keeping with a reduction in precapillary resistance at this stage [59].
Figure 4.
Capillary pressure in men and women, age < 50 years (P < 0.001).
The effects of vasoactive agents on the healthy microcirculation
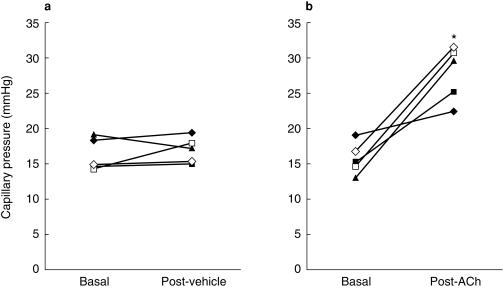
Pharmacological investigations of the healthy capillary bed, particularly those using randomised double blind protocols, are rare. At rest capillary perfusion does not appear to be regulated by nitric oxide, as assessed by the intra-arterial infusion of 1, 2, and 4 μmol min −1 l-NMMA for 10 min [60]. Marked increases in capillary perfusion, capillary pressure and capillary pulse pressure amplitude [40] accompany the nailfold application of the endothelial dependent vasodilator acetylcholine suggesting a reduction in pre to post capillary resistance ratio (Figure 5). However, the mechanisms involved have not been investigated.
Figure 5.
The effect of iontophoresis of acetylcholine or vehicle alone on nailfold capillary pressure in healthy volunteers. Acetylcholine caused a significant increase, in pressure P < 0.008. (reproduced with permission from [40]).
Atrial natriuretic peptide (ANP) (5 ng kg−1 min−1) augments capillary filtration coefficient suggesting that it enhances extravasation of fluid [61], and it also has a direct effect on the permeability of the capillary wall to water in vitro [62]. Recently Houben demonstrated a reduction in nailfold CBV during a 4 h infusion of 7 ng kg−1 min−1 ANP, which, due to the increased transit time through capillaries and consequently higher plasma osmotic pressure, would tend to offset any water extravasation [63].
Capillaroscopy and capillary pressure in disease
Morphological investigations: a role in the clinic?
Although marked morphological abnormalities occur in a variety of disease states, the data are difficult to interpret since similar changes occur following local trauma to the hands as occurs in individuals engaged in manual work such as builders or farmers. It is therefore unlikely that any morphological changes observed will be diagnostic for a particular condition. The exception is systemic sclerosis where giant capillaries are characteristic, not all loops are increased in size, microvessels of normal diameter often coexist with definately enlarged (> 20 μm) or giant capillaires (> 50 μm). Studies have shown that 82–86% of patients with progressive systemic sclerosis exhibited typical capillary changes [64, 65], Thus in a patient presenting with Raynaud's phenomenon, capillary morphological analysis may assist in the classification of the patient as primary Raynaud's or Raynaud's secondary to systemic sclerosis. Another disease in which capillary morphological analysis has proved useful clinically is in peripheral arterial occlusive disease (see later).
Capillary density, red cell velocity and pressure in disease states
This is by no means an exhaustive list of all the diseases investigated.
1 Diabetes
Capillary density
Skin capillary density is not altered in type 2 diabetes, or subjects with impaired glucose tolerance compared with age, sex and BMI matched controls [31, 66], and in the kidney no decrease in glomerular number was found in either type 2 patients or type 1 patients with normal renal function [67].
Capillary pressure
Capillary pressure is increased in patients with type 1 diabetes, particularly in those with poor glycaemic control or those with incipient nephropathy [68] suggesting that it may be a factor in the pathogenesis of diabetic microangiopathy. In normotensive normoalbuminuric type 2 patients capillary pressure is not raised [69]. However, there is a good correlation between capillary pressure and systemic blood pressure suggesting abnormalities in the normal regulatory processes.
Capillary blood cell velocity
In Type 1 diabetes, abnormalities of skin capillary flow autoregulation have been described within the first year of diabetic life. Such abnormalities are more marked with increasing disease duration particularly when accompanied by poor glycaemic control [37, 70]. Even in those patients with long disease duration without any evidence of clinical complications some degree of microvascular abnormality appears inevitable [71].
Abnormalities of capillary perfusion may be more marked in the feet than in the hands [72]. Jorneskog and colleagues suggest that abnormal capillary regulation (e.g. toe nailfold CBV is severely reduced during reactive hyperaemia in the diabetic group) may contribute to the higher risk for the development of ischaemic foot ulcers in diabetic patients with peripheral vascular disease compared with nondiabetic peers [73]. One hypothesis for the formation of neurogenic ulcers suggests a ‘capillary steal’ might be involved however, this now seems unlikely as capillary flow is increased not reduced in the diabetic neuropathic foot [74, 75].
In type 2 diabetes the capillary post occlusive reactive hyperaemia is impaired within the first 10 years after the presentation of diabetes and impairment is more marked in those with poor control [52].
Treatment effects
Replacement of human C-peptide in Type 1 patients leads to a redistribution in skin microvascular blood flow by increasing nutritive CBV relative to subpapillary arteriovenous shunt flow [76]. Insulin infusions, in the absence of changes in blood sugar, increase total capillary flow in patients with type 1 diabetes [77]. Low molecular weight heparin improved healing of diabetic foot ulcers and in seven of the eight patients improved nutritive capillary flow to the ulcer margin [78]. In a randomised double-blind study in healthy volunteers and type 1 patients enalapril failed to reduce capillary pressure [79] whereas in nephrotic type 1 patients an antihypertensive dose of captopril was associated with a reduction in capillary pressure [80].
2 Hypertension
Capillary density
Capillary rarefaction has been described in nailfold and forearm skin, and appears to be a structural rather than simply a functional defect [81–83]. Similar findings have been described in the conjunctival microcirculation and in other tissues [84]. This structural rarefaction precedes the development of hypertension being present in the dermal vessels of young men who have a familial predisposition to high blood pressure [32].
Capillary pressure
Using dynamic measurements of capillary pressure in untreated patients compared to matched controls, Williams reported an elevation in capillary pressure in the hypertensive group [85] thus although increases in peripheral resistance may serve to protect the capillary bed from perturbations of pressure, the increased postcapillary resistance and compliance probably contribute to the elevation in the capillary pressure. Capillary pressure has not been measured in subjects at risk of development of hypertension.
Capillary blood velocity
Capillary blood velocity may be reduced [86] or normal [87] in patients with untreated hypertension. Some workers find an inverse relationship between CBV and ambulatory blood pressure, higher blood pressure being associated with a low CBV [86]. The neurogenically induced reduction in CBV may be normal or enhanced [81, 87].
Treatment
Few studies have examined the effects of antihypertensive medication on capillary function as assessed by capillaroscopy. Neither the ACE inhibitors cilazapril or enalapril, the calcium antagonist amlodipine nor the selective T-type calcium channel blocker mibefradil changed resting CBV significantly in patients with hypertension [88–90]. This is in contrast to the 40% increase in CBV seen 10 min after the sublingual administration of nifedipine to healthy controls [91]. Twelve weeks treatment with enalapril did lower the immediate fall in CBV with local cooling but this effect was not sustained over the entire post cooling period, and the authors suggest this finding is probably not clinically significant [90]. In a comparison of carvedilol (α1/β-adrenoceptor blocker) and bisoprolol (β1-adrenoceptor blocker), carvedilol increased capillary flow and skin oxygen tension in healthy smokers and in hypertensive patients although this effect was not long lasting [92].
3 Peripheral arterial occlusive disease (PAOD)
Capillary density
In patients with mild disease, the capillary bed of the forefoot does not seem to be markedly abnormal however, with the advent of moderate or severe occlusion profound abnormalities of the skin capillaries have been reported. Fagrell & Lundberg [93] reported a classification system for foot capillaries which was predictive for the development of necrosis and was much more sensitive than systolic blood pressure at the toe. The final stage (Stage C-a marked reduction in capillary density or no visible capillaries), has a very high predictive value (96%) for the risk of developing skin necrosis in an area over a 3 month period. It has enabled the mapping of a foot to indicate areas ‘at risk’. Lamah and colleagues recently confirmed a reduced capillary density on both feet in subjects with unilateral rest pain or ischaemic ulcers compared to those with intermittent claudication [94].
Capillary pressure and capillary blood velocity
Capillary pressure has not been assessed in the foot of patients with PAOD. The post occlusive reactive hyperaemia for CBV is markedly impaired in the PAOD group, however CBV at rest is similar to controls. As total skin flow is increased a maldistribution of flow in favour of non-nutritional skin vessels as opposed to nutritive vessels has been suggested. In healthy capillaries rhythmic variations of CBV are observed, so called flow motion [95]. In both the fingers and toes of patients with PAOD flow motion is decreased although when present the frequency is similar (4.5–5.5 cycles min−1 [96]). Capillaroscopy with fluorescent dyes has been utilized a great deal in PAOD (for review see Bollinger [97]). Such studies have revealed increased transcapillary diffusion of NaF and inhomogeneous microvascular perfusion.
In critical ischaemia, CBV and total skin perfusion are reduced and there is a total lack of reactive hyperaemia [98]. In combination nailfold capillaroscopy, transcutaneous oxygen pressure and laser Doppler perfusion measurements measured at rest and following reactive hyperaemia appear useful in detecting nonreconstructable critical ischaemia that requires amputation which is not detected by blood pressure or clinical indicators. In their study 83% of patients with capillary density < 20/mm2, absent reactive hyperaemia and transcutaneous oxygen pressure less than 10 mmHg required amputation in the next 12 months [99].
Treatment
Pharmacotherapy may increase the number of blood filled capillaries per unit area of skin. Fagrell showed this to be the case using PGE1 and there was good agreement between the disappearance of rest pain and the increased capillary density [100]. Spinal cord stimulation can also improve capillary density and microcirculatory reactive hyperaemia [101, 102]. In a further study the skin microcirculation was examined before and after treatment with either arteriography or percutaneous transluminal angioplasty (PTA) in matched groups of patients. Nutritional (capillary) flow was increased in the arteriography group but the increased blood flow in the PTA group was primarily non-nutritive [103].
Discussion
Capillaroscopy has been used successfully to monitor skin microcirculatory changes in health and disease. Although until recently it has primarily been a research tool, its use in the differential diagnosis of some diseases (e.g. systemic sclerosis) has long been acknowledged. It's use in the assessment of treatment for peripheral arterial occlusive disease [102] demonstrates it capabilities and simple challenge response testing (e.g. reactive hyperaemia) is becoming increasingly common as a clinical tool.
To date capillaroscopy and the measurement of capillary pressure have not been used a great deal in pharmacology. The improvements in automated analysis of capillary red blood cell velocity and capillary density, the novel drug delivery systems which allow drug delivery without perturbation of the local skin microcirculation, the increasing availablility of specific blockers of the pathways involved in the control of the microcirculation, and the growing recognition of the importance of the microcirculatory bed for tissue health make it likely that this will be a rapidly growing area in the future. The measurement of capillary pressure is likely to remain a research tool. However in combination with simultaneously measured capillary flow, the local application of pharmacological agonists and antagonists, and the introduction of ion selective, O2 or NO microelectrodes, unique data regarding the control of capillary pressure in man may be obtained.
Normal capillary function is essential for tissue homeostasis. Other microcirculatory techniques measure skin perfusion but none is able to monitor the nutritive component passing through capillaries. Direct monitoring of a capillary using capillaroscopy provides a unique noninvasive method of investigating capillary function in health and in disease states. Furthermore the recent observation that skin melanoma microvasculature is visible using capillaroscopy, and may provide an in vivo tumour microcirculation model [104], raises the exciting prospect of looking at tumour vascular control and the effects of anticancer therapy on angiogeneis and vascular reactivity, in vivo.
References
- 1.Michel CC. Starling: the formulation of his hypothesis of microvascular fluid exchange and its significance after 100 years. Exp Physiol. 1997;82:1–30. doi: 10.1113/expphysiol.1997.sp004000. [DOI] [PubMed] [Google Scholar]
- 2.Levick JR. An introduction to cardiovascular physiology. London: Butterworth and Co Ltd; 1991. ISBN 0-750-61028-X. [Google Scholar]
- 3.Koller A, Sun D, Kaley G. Role of shear stress and endothelial prostaglandins in flow- and viscosity-induced dilation of arterioles in vitro. Circulation Res. 1993;72:1276–1284. doi: 10.1161/01.res.72.6.1276. [DOI] [PubMed] [Google Scholar]
- 4.de Wit C, von Bismarck P, Pohl U. Mediator role of prostaglandins in acetylcholine-induced vasodilation and control of resting vascular diameter in the hamster cremaster microcirculation in vivo. J Vascular Res. 1993;30:272–278. doi: 10.1159/000159006. [DOI] [PubMed] [Google Scholar]
- 5.Tang T, Joyner WL. Differential role of endothelial function on vasodilator responses in series-arranged arterioles. Microvascular Res. 1992;44:61–72. doi: 10.1016/0026-2862(92)90102-u. [DOI] [PubMed] [Google Scholar]
- 6.Wolf S, Arend O, Toonen H, Bertram B, Jung F, Reim M. Retinal capillary blood flow measurement with a scanning laser opthalmoscope. Ophthalmology. 1991;98:996–1000. doi: 10.1016/s0161-6420(91)32192-4. [DOI] [PubMed] [Google Scholar]
- 7.Parazynski SE, Tucker BJ, Aratow M, Crenshaw A, Hargens AR. Direct measurement of capillary blood pressure in the human lip. J Appl Physiol. 1993;74:946–950. doi: 10.1152/jappl.1993.74.2.946. [DOI] [PubMed] [Google Scholar]
- 8.Fagrell B, Fronek A, Intaglietta M. A microscope-television system for studying flow velocity in human skin capillaries. Am J Physiol. 1977:H318–H321. doi: 10.1152/ajpheart.1977.233.2.H318. [DOI] [PubMed] [Google Scholar]
- 9.Braverman IM. The cutaneous microcirculation: Ultrastructure and microanatomical organisation. Microcirculation. 1997;4:329–340. doi: 10.3109/10739689709146797. [DOI] [PubMed] [Google Scholar]
- 10.Lamah M, Mortimer PS, Dormandy JA. Heterogeneity of capillary density of skin over the dorsum of the foot and toes of healthy subjects. Int J Microcirculation: Clin Exp. 1996;16:271–276. doi: 10.1159/000179184. [DOI] [PubMed] [Google Scholar]
- 11.Kelly RI, Pearse R, Bull RH, Leveque JL, de Rigal J, Mortimer PS. The effects of aging on the cutaneous microvasculature. J Am Acad Dermatol. 1995;33:749–756. doi: 10.1016/0190-9622(95)91812-4. [DOI] [PubMed] [Google Scholar]
- 12.Montagna W, Carlisle K. Structural changes in aging human skin. J Invest Derm. 1979;73:47–53. doi: 10.1111/1523-1747.ep12532761. [DOI] [PubMed] [Google Scholar]
- 13.Malphigi M. 1661. In De Pulmonibus Observationes Anatomicae.
- 14.Heuter C. Die Cheilo Angioskopie, eine neue Untersuchungsmethode zu physiologischen. ZBL. Med Iss. 1879;17:225–230. [Google Scholar]
- 15.Muller O. 1922. Die kapillaren der menschlichen Korperoberflache in gesunden und kranken Tagen. Enke, Stuttgart, Table iv Figure 5.
- 16.Basler A. Uber die Bestimmung der Stromungsgeschwindigkeit in den Blutkapilallen der menschlichen Haut. Muench Med Wochenschr. 1919;13:347–348. [Google Scholar]
- 17.Zimmer JG, Demis DJ. The study of physiology and pharmacology of the human cutaneous microcirculation by capillary microscopy and television cinematography. Angiology. 1964;15:232–235. doi: 10.1177/000331976401500505. [DOI] [PubMed] [Google Scholar]
- 18.Bollinger A, Butti P, Barras JP, et al. Red blood velocity in nailfold capillaries of man measured by a television microscopy technique. Microvasc. Res. 1974;7:61–72. doi: 10.1016/0026-2862(74)90037-5. [DOI] [PubMed] [Google Scholar]
- 19.Carrier EB, Rehberg PB. Capillary and venous pressure in man. Skand Arch Physiol. 1923;44:20–31. [Google Scholar]
- 20.Lombard WC. The blood pressure in the arterioles, capillaries and small veins of the human skin. Am J Physiol. 1912;XXIX:335–362. Bd. [Google Scholar]
- 21.Landis EM. Micro-injection studies of capillary blood pressure in human skin. Heart. 1930;15:209–228. [Google Scholar]
- 22.Eichna LW, Bordley J. Capillary blood pressure in man. Direct measurements in the digits of normal and hypertensive subjects during vasoconstriction and vasodilatation variously induced. J Clin Invest. 1942;21:711–729. doi: 10.1172/JCI101347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fahr G, Ershler I. Studies of the factors concerned in edema formation. II. The hydrostatic pressure in the capillaries during edema formation in right heart failure. Ann Intern Med. 1941. pp. 798–810.
- 24.Macleod M. Systemic capillary pressure in acute glomerulonephritis estimated by direct micropuncture. Clin Sci. 1960;19:27–33. [PubMed] [Google Scholar]
- 25.Mahler F, Muheim MH, Intaglietta M, Bollinger A, Anliker M. Blood pressure fluctuations in human nailfold capillaries. Am J Physiol. 1979;236:H888–H893. doi: 10.1152/ajpheart.1979.236.6.H888. [DOI] [PubMed] [Google Scholar]
- 26.Bollinger A, Fagrell B. In: Clinical Capillaroscopy. Bollinger A, Fagrell B, editors. Hogrefe & Huber Publishers; 1991. [Google Scholar]
- 27.Bollinger A, Junger K, Sgier F, Seglias J. Fluorescence microlymphography. Circulation. 1981;6:1195–1200. doi: 10.1161/01.cir.64.6.1195. [DOI] [PubMed] [Google Scholar]
- 28.Spiegel M, Vesti B, Shore AC, Franzeck UK, Becker F, Bollinger A. Pressure of lymphatic capillaries in human skin. Am J Physiol. 1992;262:H1208–H1210. doi: 10.1152/ajpheart.1992.262.4.H1208. [DOI] [PubMed] [Google Scholar]
- 29.Flynn MD, Williams SA, Tooke JE. Clinical television microscopy. J Med Eng Technol. 1989;13:278–284. doi: 10.3109/03091908909016202. [DOI] [PubMed] [Google Scholar]
- 30.Coleridge Smith PD, Scurr JH. Capillary microscopy demonstrates functional changes in patients with chronic venous insufficiency. Phlebologie. 1988;41:786–787. [Google Scholar]
- 31.Jaap AJ, Shore AC, Stockman AJ, Tooke JE. Skin capillary density in subjects with impaired glucose tolerance and patients with type 2 diabetes. Diabetic Med. 1996;13:160–164. doi: 10.1002/(SICI)1096-9136(199602)13:2<160::AID-DIA36>3.0.CO;2-7. 10.1002/(sici)1096-9136(199602)13:2<160::aid-dia36>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 32.Noon JP, Walker BR, Webb DJ, et al. Impaired microvascular dilatation and capillary rarefaction in young adults with a predisposition to high blood pressure. J Clin Invest. 1997;99:1873–1879. doi: 10.1172/JCI119354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Serne EH, Stehouwer CD, ter Maaten JC, et al. Microvascular function relates to insulin sensitivity and blood pressure in normal subjects. Circulation. 1999;23:896–902. doi: 10.1161/01.cir.99.7.896. 99(7) [DOI] [PubMed] [Google Scholar]
- 34.Antonios TFT, Rattray FEM, Singer DRJ, Markandu ND, Mortimer PS, MacGregor GA. Maximisation of skin capillaries during intravital video-microscopy in essential hypertension: comparison between venous congestion, reactive hyperaemia and core heat load tests. Clin Sci. 1999;97:523–528. [PubMed] [Google Scholar]
- 35.Aberne WA, Dunnill MS. Morphometry. London: Edward Arnold; 1982. Point Counting and the Estimation of Volume Fraction; pp. 33–45. [Google Scholar]
- 36.Moneta G, Brulisauer M, Jager K, Bollinger A. Infrared fluorescence video microscopy of skin capillaries with indocyanide green. Int J Microcirc: Clin Exp. 1987:25–34. ??: [PubMed] [Google Scholar]
- 37.Tooke JE, Lins P-E, Ostergren J, Fagrell B. Skin microvascular autoregulatory responses in type I diabetes: the influence of duration and control. Int J Microcirc: Clin Exp. 1985;4:249–256. [PubMed] [Google Scholar]
- 38.Mahler F, Sanner H, Annaheim M, Linder HR. Capilloroscopic evaluation of erythrocyte flow velocity in patients with raynaud's syndrome by means of a local cold exposure test. Prog Appl Microcirc. 1986;11:47–59. [Google Scholar]
- 39.Hahn M, Shore AC. The effect of rapid local cooling on human finger nailfold capillary blood pressure and blood cell velocity. J Physiol (Lond) 1994;478:109–114. doi: 10.1113/jphysiol.1994.sp020234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Morris SJ, Kunzek S, Shore AC. The effect of acetylcholine on finger capillary pressure and capillary flow in healthy volunteers. J Physiol. 1996;494:307–313. doi: 10.1113/jphysiol.1996.sp021493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gasser P. Capillary blood cell velocity in finger nailfold: Characteristics and reproducibility of the local cold response. Microvascular Res. 1990;40:29–35. doi: 10.1016/0026-2862(90)90004-b. [DOI] [PubMed] [Google Scholar]
- 42.Hahn M, Klyscz T, Bohnenberger G, Junger M. A new technique for controlling the finger skin temperature in microcirculatory research demonstrated in a local cold stress test in healthy controls and patients with Raynaud's phenomenon. Int J Microcirc: Clin Exp. 1996;16:16–22. doi: 10.1159/000179146. [DOI] [PubMed] [Google Scholar]
- 43.Ostergren J, Fagrell B. Skin capillary blood velocity in man. Characteristics and reproducibility of the reactive hyperaemic response. Int J Microcirc: Clin Exp. 1986;5:37–51. [PubMed] [Google Scholar]
- 44.Ubbink DTh. Netherlands: Maastricht; 1992. On skin microvascular reactivity in patients with lower limb ischaemia. ISBN 90–9005152-X, Thesis. [DOI] [PubMed] [Google Scholar]
- 45.Branemark P-I, Jonsson I. Determination of the Velocity of corpuscles in blood capillaries. Biorheology. 1963;1:143–146. [Google Scholar]
- 46.Rosen L, Silverman NR. Videodensitometric measurement of blood flow using crosscorrelation techniques. Radiology. 1973;109:305–310. doi: 10.1148/109.2.305. [DOI] [PubMed] [Google Scholar]
- 47.Tompkins WR, Monti R, Intaglietta M. Velocity measurement by self-tracking correlator. Rev Sci Instrum. 1974;45:647–649. [Google Scholar]
- 48.Fagrell B, Eriksson SE, Malmstrom S, Sjolund A. Computerised data analysis of capillary blood cell velocity. Int J Microcirc: Clin Exp. 1988;7:276. [Google Scholar]
- 49.Mawson DM, Shore AC. Comparison of capiflow and frame by frame analysis for the assessment of capillary red blood cell velocity. J Med Engineering Technol. 1998;22:53–63. doi: 10.3109/03091909809010000. [DOI] [PubMed] [Google Scholar]
- 50.Lentner A, Berger F, Wienert V. [‘Spatial shift alignment (SSA)’ a new method for determining blood flow velocity in video capillary microscopy] Das ‘Spatial Sheft Alignment (SSA)’ eine neue Methode zur Mestimmung der Blutflussgeschwindigkeit in der Video-Kapillarmikroskopie. Biomedizinische Technik (Berlin) 1994;39:170–175. doi: 10.1515/bmte.1994.39.7-8.170. [DOI] [PubMed] [Google Scholar]
- 51.Stucker M, Baier V, Reuther T, Hoffmann K, Kellam K, Altmeyer P. Capillary blood cell velocity in human skin capillaries located perpendicularly to the skin surface: measured by a new laser Doppler anemometer. Microvascular Res. 1996;52:188–192. doi: 10.1006/mvre.1996.0054. 10.1006/mvre.1996.0054. [DOI] [PubMed] [Google Scholar]
- 52.Meyer MF, Schatz H. Influence of metabolic control and duration of disease on microvascular dysfunction in diabetes assessed by laser Doppler anemometry. Exp Clin Endocrinol Diabetes. 1998;106:395–403. doi: 10.1055/s-0029-1212005. [DOI] [PubMed] [Google Scholar]
- 53.Weiderhielm CA, Weston BV. Microvascular, lymphatic, and tissue pressures in the unanesthetized mammal. Am J Physiol. 1973;225:992–996. doi: 10.1152/ajplegacy.1973.225.4.992. [DOI] [PubMed] [Google Scholar]
- 54.Intaglietta M, Pawula RF, Tompkins WR. Pressure measurements in the mammalian microvasculature. Microvascular Res. 1970;2:212–220. doi: 10.1016/0026-2862(70)90009-9. [DOI] [PubMed] [Google Scholar]
- 55.Shore AC, Sandeman DD, Tooke JE. Capillary pressure pulse pressure amplitude and pressure waveform in healthy volunteers. Am J Physiol. 1995;268:H147–H154. doi: 10.1152/ajpheart.1995.268.1.H147. [DOI] [PubMed] [Google Scholar]
- 56.Mahy IR, Tooke JE, Shore AC. Effect of incremental venous pressure elevation on capillary pressure in normal volunteers. Int J Microcirc: Clin Exp. 1994;14:245. [Google Scholar]
- 57.Levick JR, Michel CC. The effects of position and skin temperature on the capillary pressures in the fingers and toes. J Physiol (Lond) 1978;274:97–109. doi: 10.1113/jphysiol.1978.sp012136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shore AC, Sandeman DD, Tooke JE. Effect of an increase in systemic blood pressure on nailfold capillary pressure in humans. Am J Physiol. 1993;265:H820–H823. doi: 10.1152/ajpheart.1993.265.3.H820. [DOI] [PubMed] [Google Scholar]
- 59.Hahn M, Shore AC. The effect of rapid local cooling on human finger nailfold capillary blood pressure and blood cell velocity. J Physiol (Lond) 1994;478:109–114. doi: 10.1113/jphysiol.1994.sp020234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Noon JP, Haynes WG, Webb DJ, Shore AC. Local inhibition of nitric oxide generation in man reduces blood flow in finger pulp but not in hand dorsum skin. J Physiol. 1996;490:501–508. doi: 10.1113/jphysiol.1996.sp021161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Groban L, Cowley Aw, Jr, Ebert TJ. Atrial natriuretic peptide augments forearm capillary filtration in humans. Am J Physiol. 1990;259:H258–H263. doi: 10.1152/ajpheart.1990.259.1.H258. [DOI] [PubMed] [Google Scholar]
- 62.Meyer DJ, Jr, Huxley VH. Differential sensitivity of exhange vessel hydraulic conductivity to atrial natriuretic peptide. Am J Physiol. 1990;258:H521–H528. doi: 10.1152/ajpheart.1990.258.2.H521. [DOI] [PubMed] [Google Scholar]
- 63.Houben AJ, Krekels MM, Schaper NC, Fuss-Lejeune MJ, de Rodriguez SA, Leeuw PW. Microvascular effects of atrial natriuretic peptide (ANP) in man: studies during high and low salt diet. Cardiovascular Res. 1998;39:442–450. doi: 10.1016/s0008-6363(98)00072-8. [DOI] [PubMed] [Google Scholar]
- 64.Maricq HR, LeRoy EC, D'angelo WA, et al. Diagnostic potential of in vivo capillary microscopy in scleroderma and related disorders. Arthritis Rheum. 1980;23:183–189. doi: 10.1002/art.1780230208. [DOI] [PubMed] [Google Scholar]
- 65.Lovy M, MacCarter D, Steigerwald JC. Relationship between nailfold capillary abnormalities and organ involvement in systemic sclerosis. Arthritis Rheum. 1985;28:496–501. doi: 10.1002/art.1780280505. [DOI] [PubMed] [Google Scholar]
- 66.Katz MA, McCuskey P, Beggs JL, Johnson PC, Gaines JA. Relationships between microvascular function and capillary structure in diabetic and nondiabetic human skin. Diabetes. 1989;38:1245–1250. doi: 10.2337/diab.38.10.1245. [DOI] [PubMed] [Google Scholar]
- 67.Bendtsen TF, Nyengaard JR. The number of glomeruli in type 1 (insulin-dependent) and type 2 (non-insulin-dependent) diabetic patients. Diabetologia. 1992;35:844–850. doi: 10.1007/BF00399930. [DOI] [PubMed] [Google Scholar]
- 68.Sandeman DD, Shore AC, Tooke JE. Relation of skin capillary pressure in patients with insulin-dependent diabetes mellitus to complications and metabolic control. N Engl J Med. 1992;327:760–764. doi: 10.1056/NEJM199209103271103. [DOI] [PubMed] [Google Scholar]
- 69.Shore AC, Jaap AJ, Tooke JE. Capillary pressure in patients with NIDDM. Diabetes. 1994;43:1198–1202. doi: 10.2337/diab.43.10.1198. [DOI] [PubMed] [Google Scholar]
- 70.Jorneskog G, Brismar K, Fagrell B. Pronounced skin capillary ischemia in the feet of diabetic patients with bad metabolic control. Diabetologia. 1998;41:410–415. doi: 10.1007/s001250050923. 10.1007/s001250050923. [DOI] [PubMed] [Google Scholar]
- 71.Tooke JE, Ostergren J, Lins P-E, Fagrell B. Skin microvascular blood flow control in long duration diabetics with and without complications. Diabetes Res. 1987;5:189–192. [PubMed] [Google Scholar]
- 72.Jorneskog G, Fagrell B. Discrepency in skin capillary circulation between finger and toes in patients with type 1 diabetes. Int J Microcirc: Clin Exp. 1996;16:313–319. doi: 10.1159/000179191. [DOI] [PubMed] [Google Scholar]
- 73.Jorneskog G, Brismar K, Fagrell B. Skin capillary circulation is more impaired in the toes of diabetic than non-diabetic patients with peripheral vascular disease. Diab Med. 1995;12:36–41. doi: 10.1111/j.1464-5491.1995.tb02059.x. [DOI] [PubMed] [Google Scholar]
- 74.Netten PM, Wollersheim H, Thien T, Lutterman JA. Skin microcirculation of the foot in diabetic neuropathy. Clin Sci. 1996;91:559–565. doi: 10.1042/cs0910559. [DOI] [PubMed] [Google Scholar]
- 75.Flynn MD, Edmonds ME, Tooke JE, Watkins PJ. Direct measurement of capillary blood flow in the diabetic neuropathic foot. Diabetologia. 1988;31:652–656. doi: 10.1007/BF00278747. [DOI] [PubMed] [Google Scholar]
- 76.Forst T, Kunt T, Pohlmann T, et al. Biological activity of C-peptide on the skin microcirculation in patients with insulin-dependent diabetes mellitus. J Clin Invest. 1998;101:2036–2041. doi: 10.1172/JCI2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tooke JE, Lins PE, Ostergren J, Adamson U, Fagrell B. The effects of intravenous insulin infusion on skin microcirculatory flow in type 1 diabetes. Int J Microcirc: Clin Exp. 1985;69:69–83. [PubMed] [Google Scholar]
- 78.Jorneskog G, Brismar K, Fagrell B. Low molecular weight heparin seems to improve local capillary circulation and healing of chronic foot ulcers in diabetic patients. VASA. 1993;22:137–142. [PubMed] [Google Scholar]
- 79.Shore AC, Sandeman DD, Tooke JE. Nailfold capillary pressure in non-nephropathic diabetic patients of moderate disease duration: the effect of angiotensin converting enzyme inhibition by enalapril. Int. J Microcirc: Clin Exp. 1992;11:445. [Google Scholar]
- 80.Sandeman DD, Shore AC, Tooke JE. Captopril reduces nailfold capillary pressure in type 1 diabetic patients with established nephropathy. Diabetic Med. 1991;8(Suppl 1):37A. [Google Scholar]
- 81.Gasser P, Buhler FR. Nailfold microcirculation in normotensive and essential hypertensive subjects, as assessed by video-microscopy. J Hypertension. 1992;10:83–86. doi: 10.1097/00004872-199201000-00013. [DOI] [PubMed] [Google Scholar]
- 82.Prasad A, Dunhill GS, Mortimer PS, MacGregor GA. Capillary rearefaction in the forearm skin in essential hypertension. J Hypertension. 1995;13:265–268. [PubMed] [Google Scholar]
- 83.Antonios TFT, Singer DRJ, Markandu Nd Mortimer PS, Macgregor GA. Structural skin capillary rarefaction in essential hypertension. Hypertension. 1999;33:998–1001. doi: 10.1161/01.hyp.33.4.998. [DOI] [PubMed] [Google Scholar]
- 84.Shore AC, Tooke JE. Microvascular function in human essential hypertension. J Hypertension. 1994;12:717–728. [PubMed] [Google Scholar]
- 85.Williams SA, Boolell M, MacGregor GA, Smaje LH, Wasserman SM, Tooke JE. Capillary hypertension and abnormal pressure dynamics in patients with essential hypertension. Clin Sci. 1990;79:5–8. doi: 10.1042/cs0790005. [DOI] [PubMed] [Google Scholar]
- 86.Duprez D, De Buyzere M, De Backer T, Vercammen J, Brusselmans F, Clement DL. Impaired microcirculation in mild-to-moderate essential arterial hypertension. J Hypertension. 1992;10:251–254. doi: 10.1097/00004872-199203000-00008. [DOI] [PubMed] [Google Scholar]
- 87.Ostergren J, Kahan T, Hjemdahl P, Fagrell B, de Faire U, Lindvall K. Effects of sympatho-adrenal activation on the finger microcirculation in mild hypertension. J Hum Hypertension. 1992;6:169–173. [PubMed] [Google Scholar]
- 88.Weinbacher M, Martina B, Gasser P, Kohler M, Bart T. Nailfold capillaroscopy and echocardiography in mild to moderate hypertension treated with cilazapril plus hydrochlorothiazide: first results. J Cardiovasc Pharmacol. 1994;24(Suppl 3):S83–S85. [PubMed] [Google Scholar]
- 89.Martina B, Weinbacher M, Drewe J, Gasser P. Effect of losartan titrated to losartan/hydrochlorothiazide and amlodipine on blood pressure and peripheral capillary microcirculation in patients with mild to moderate hypertension. J Human Hypertension. 1998;12:473–478. doi: 10.1038/sj.jhh.1000647. [DOI] [PubMed] [Google Scholar]
- 90.Martina B, Frach B, Surber C, Drewe J, Battegay E, Gasser P. Capillary blood cell velocity in finger nailfold: effect of enalapril and mibefradil in patients with mild to moderate hypertension. Microvascular Res. 1999;57:94–99. doi: 10.1006/mvre.1998.2125. 10.1006/mvre.1998.2125. [DOI] [PubMed] [Google Scholar]
- 91.Ostergren J, Fagrell B. Videophotometric capillaroscopy for evaluating drug effects on skin microcirculation – a double blind study with nifedipine. Clin Phys. 1984;4:169–176. doi: 10.1111/j.1475-097x.1984.tb00232.x. [DOI] [PubMed] [Google Scholar]
- 92.Albrecht M, Haustein KO. The effects of 2 beta-receptor blocking agents on the microcirculation of healthy subjects and of hypertensive patients. Int J Clin Pharmacol Ther. 1997;35:580–586. [PubMed] [Google Scholar]
- 93.Fagrell B, Lundberg G. A simplified evaluation of vital capillary microscopy for predicting skin viability in patients with severs arterial insufficiency. Clin Physiol. 1984;4:403–411. doi: 10.1111/j.1475-097x.1984.tb00125.x. [DOI] [PubMed] [Google Scholar]
- 94.Lamah M, Mortimer PS, Dormandy JA. Quantitative study of capillary density in the skin of the foot in peripheral vascular disease. Br J Surgery. 1999;86:342–348. doi: 10.1046/j.1365-2168.1999.01039.x. 10.1046/j.1365-2168.1999.01039.x. [DOI] [PubMed] [Google Scholar]
- 95.Bongard O, Fagrell B. Variations in laser doppler flux and flow motion patterns in the dorsal skin of the human foot. Microvasc Res. 1990;39:212–222. doi: 10.1016/0026-2862(90)90071-x. [DOI] [PubMed] [Google Scholar]
- 96.Bongard O, Fagrell B. Discrepancies between total and nutritional skin microcirculation in patients with peripheral arterial occlusive disease (PAOD) VASA. 1990;19:105–111. [PubMed] [Google Scholar]
- 97.Bollinger A, Hoffmann U, Franzeck UK. Microvascular changes in arterial occlusive disease: target for pharmacotherapy. Vascular Med. 1996;1:50–54. doi: 10.1177/1358863X9600100109. [DOI] [PubMed] [Google Scholar]
- 98.Hoffman U, Franzeck UK, Geiger M, Yanar A, Bollinger A. Variability of different patterns of skin oscillatory flux in healthy controls and patients with peripheral arterial occlusive disease. Int J Microcirc: Clin Exp. 1993;12:255–273. [PubMed] [Google Scholar]
- 99.Ubbink DT, Spincemaille GH, Reneman RS, Jacobs MJ. Prediction of imminent amputation in patients with non-reconstructible leg ischaemia by means of microcirculatory investigations. J Vasc Surg. 1999;30:114–121. doi: 10.1016/s0741-5214(99)70183-7. [DOI] [PubMed] [Google Scholar]
- 100.Fagrell B, Lundberg G, Olsson A, Ostergren J. PGE1 treatment of severe skin ischemia in patients with peripheral arterial insufficiency – the effect on skin microcirculation. VASA. 1986;15:56–60. [PubMed] [Google Scholar]
- 101.Jacobs M, Jorning P, Joshi S, Kitslaar P, Slaaf D, Reneman R. Epidural spinal cord electrical stimulation improves microvascular blood flow in severe limb ischaemia. Ann Surg. 1988;207:179–183. doi: 10.1097/00000658-198802000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ubbink DT, Spincemaille GH, Prins MH, Reneman RS, Jacobs MJ. Microcirculatory investigations to determine the effects of spinal cord stimulation for critical leg ischaemia: the Dutch multicenter randomised controlled trial. J Vasc Surg. 1999;30:236–244. doi: 10.1016/s0741-5214(99)70133-3. [DOI] [PubMed] [Google Scholar]
- 103.Bongard O, Didier D, Bounameaux H. Effects of percutaneous transluminal angioplasty on skin microcirculation in patients with disabling peripheral arterial occlusive disease. Int J Microcirc: Clin Exp. 1994;14:319–326. doi: 10.1159/000178850. [DOI] [PubMed] [Google Scholar]
- 104.Bedlow AJ, Stanton AW, Cliff S, Mortimer PS. Basal cell carcinoma—an in-vivo model of human tumour microcirculation? Exp Dermatol. 1999;3:222–226. doi: 10.1111/j.1600-0625.1999.tb00374.x. [DOI] [PubMed] [Google Scholar]