Abstract
Aims
To evaluate whether ketoconazole or cimetidine alter the pharmacokinetics of loratadine, or its major metabolite, desloratadine (DCL), or alter the effects of loratadine or DCL on electrocardiographic repolarization in healthy adult volunteers.
Methods
Two randomized, evaluator-blind, multiple-dose, three-way crossover drug interaction studies were performed. In each study, subjects received three 10 day treatments in random sequence, separated by a 14 day washout period. The treatments were loratadine alone, cimetidine or ketoconazole alone, or loratadine plus cimetidine or ketoconazole. The primary study endpoint was the difference in mean QTc intervals from baseline to day 10. In addition, plasma concentrations of loratadine, DCL, and ketoconazole or cimetidine were obtained on day 10.
Results
Concomitant administration of loratadine and ketoconazole significantly increased the loratadine plasma concentrations (307%; 90% CI 205–428%) and DCL concentrations (73%; 62–85%) compared with administration of loratadine alone. Concomitant administration of loratadine and cimetidine significantly increased the loratadine plasma concentrations (103% increase; 70–142%) but not DCL concentrations (6% increase; 1–11%) compared with administration of loratadine alone. Cimetidine or ketoconazole plasma concentrations were unaffected by coadministration with loratadine. Despite increased concentrations of loratadine and DCL, there were no statistically significant differences for the primary electrocardiographic repolarization parameter (QTc) among any of the treatment groups. No other clinically relevant changes in the safety profile of loratadine were observed as assessed by electrocardiographic parameters (mean (90% CI) QTc changes: loratadine vs loratadine + ketoconazole = 3.6 ms (−2.2, 9.4); loratadine vs loratadine + cimetidine = 3.2 ms (−1.6, 7.9)), clinical laboratory tests, vital signs, and adverse events.
Conclusions
Loratadine 10 mg daily was devoid of any effects on electrocardiographic parameters when coadministered for 10 days with therapeutic doses of ketoconazole or cimetidine in healthy volunteers. It is concluded that, although there was a significant pharmacokinetic drug interaction between ketoconazole or cimetidine and loratadine, this effect was not accompanied by a change in the QTc interval in healthy adult volunteers.
Keywords: cimetidine, electrocardiographic, ketoconazole, loratadine, repolarization
Introduction
Cytochrome P450 enzymes are responsible for the metabolism of many drugs, including the nonsedating antihistamines loratadine, terfenadine, and astemizole. Several clinically important drug interactions involve coadministration of terfenadine and astemizole with drugs that inhibit their metabolism by cytochrome P450 enzymes [1–9]. For example, coadministration of ketoconazole with terfenadine has been associated with altered cardiac repolarization leading to QTc interval prolongation and torsades de pointes [2, 7]. Similarly, ventricular dysrhythmias and torsades de pointes have been reported with astemizole, usually with overdosage. These clinical findings have led to label warnings [9], and in the case of terfenadine, removal from the market in United States and France.
Loratadine is a selective peripheral H1-receptor antagonist that is active following oral administration and requires only once daily dosing. The efficacy of loratadine for the relief of symptoms associated with seasonal allergic rhinitis as well as for the treatment of chronic idiopathic urticaria, and its lack of undesirable side-effects have been demonstrated in several well-controlled clinical trials.
The metabolism of loratadine to DCL involves cytochrome P450 3A4 and, to a lesser extent, cytochrome P450 2D6 [10]. The safety of loratadine when coadministered with the P450 3A4 inhibitor erythromycin has been shown previously [11]. Because ketoconazole is a potent inhibitor of P450 3A4 and cimetidine inhibits both P450 3A4 and P450 2D6 [12, 13], two clinical studies were designed to evaluate the effects of coadministration of loratadine plus ketoconazole or cimetidine.
Methods
Study design
Two randomized, evaluator-blind, multiple-dose, three-way crossover drug interaction studies were conducted. Aside from the active treatments, both studies followed identical protocols. Prior to initiation of either study, the protocol and statement of informed consent were approved by the clinical site's Institutional Review Board, and written informed consent was obtained from each volunteer. Steps taken to assure accurate and reliable data included selection of an established investigator and study centre, and monitoring by the sponsor in accordance with the United States Food and Drug Administration guidelines.
In each study, volunteers received three 10 day treatments in random sequence, separated by a 14 day washout period. Treatment assignments for the ketoconazole study were as follows:
Treatment A
One loratadine 10 mg tablet once daily in the morning (07.00 h) on an empty stomach and one ketoconazole 200 mg tablet every 12 h (07.00 h and 19.00 h) for 10 days
Treatment B
One loratadine 10 mg tablet once daily in the morning (07.00 h) on an empty stomach and one placebo tablet every 12 h (07.00 h and 19.00 h) for 10 days
Treatment C
One placebo tablet once daily in the morning (07.00 h) on an empty stomach and one ketoconazole 200 mg tablet every 12 h (07.00 h and 19.00 h) for 10 days
Treatment assignments for the cimetidine study were as follows:
Treatment D
One loratadine 10 mg tablet once daily in the morning (07.00 h) on an empty stomach and one cimetidine 300 mg tablet four times daily (09.00 h, 13.00 h, 18.00 h, and 23.00 h) for 10 days
Treatment E
One loratadine 10 mg tablet once daily in the morning (07.00 h) on an empty stomach and one placebo tablet four times daily (09.00 h, 13.00 h, 18.00 h, and 23.00 h) for 10 days
Treatment F
One placebo tablet once daily in the morning (07.00 h) on an empty stomach and one cimetidine 300 mg tablet four times daily (09.00 h, 13.00 h, 18.00 h, and 23.00 h) for 10 days
Loratadine (Claritin®) 10 mg tablets and placebo tablets were manufactured by Schering-Plough Corporation, Kenilworth, NJ. The tablets were supplied in commercial bottles and were not repackaged. The ketoconazole (Nizoral®) 200 mg tablets were manufactured by Janssen Pharmaceuticals, Titusville, NJ, and the cimetidine (Tagamet®) 300 mg tablets were manufactured by SmithKline Beecham, Philadelphia, PA.
Because the dosage forms used in this study differed, a third-party assigned volunteers to the various treatment groups according to a random code and supervised the administration of assigned treatments to each volunteer. The third-party was not the investigator and did not divulge the treatment assignments to the investigator or his staff. Individuals involved in the clinical evaluation were not allowed to observe dosage administration.
Study volunteers were confined to the study site at least 36 h prior to the initial treatment administration for each treatment phase and remained confined until after the ECG and clinical laboratory safety tests were obtained 48 h after the 07.00 h dose on day 10. Laboratory tests performed at screening (including serum magnesium and the urine screen for drugs with a high potential for abuse, but excluding HIV, hepatitis B, and hepatitis C-tests) were repeated. A light snack (sandwich, fruit, and caffeine-free beverage) was served between 9 and 10 h prior to dosing, after which an overnight fast (no fluids except water) was maintained. All treatments were administered with 6 fluid ounces of tap water. All meals were standardized throughout the confinement periods. Because grapefruit and grapefruit juice are known to alter first-pass metabolism of some antihistamines, they were not allowed for the duration of the study.
Study population
Twenty-five healthy adult male volunteers between the ages of 18 and 40 years (mean 32.8 years) and weighing between 135 and 224 lbs (mean 165.5 lbs) participated in the ketoconazole study. Twenty-four healthy adult male volunteers between the ages of 19 and 39 years (mean 33.6 years) and weighing between 138 and 209 lbs (mean 167.0 lbs) participated in the cimetidine study. All volunteers had body weights in accordance with current actuarial tables (± 10%) and were determined to be in good health on the basis of medical history, physical examination, ECG and routine laboratory safety tests (complete blood count, blood chemistry and urinalysis). All subjects tested negative on a urine screen for drugs with a high potential for abuse, for hepatitis B surface antigen, hepatitis C antibody, and for infection with HIV. None of the study volunteers used either terfenadine (Seldane®, Hoechst Marion Roussel, Kansas City, MO) or astemizole (Hismanal®, Janssen Pharmaceuticals, Titusville, NJ) within the preceding 90 days.
Pharmacokinetic analysis
Plasma samples were obtained for determination of loratadine, DCL, and ketoconazole or cimetidine concentrations immediately prior to the morning treatment administration (07.00 h) on days 7, 8, and 9, as well as on day 10 prior to the 07.00 h treatment administration and at 0.5, 1, 1.5, 2, 3, 4, 6, 8, 12, 16, and 24 h after the dose.
The concentrations of loratadine and DCL in plasma were determined by validated gas chromatographic (GC) methods with a lower limit of quantification (LOQ) of 0.1 ng ml−1[15]. The concentrations of cimetidine in plasma were determined by a validated high performance liquid chromatographic (h.p.l.c.) method with an LOQ of 0.05 µg ml−1. These analyses were performed at Wisconsin Analytical Research Services, Ltd, Madison, WI. The concentrations of ketoconazole in plasma were determined by a validated h.p.l.c. method with an LOQ of 0.05 µg ml−1 at Bioassay Laboratory, Inc., Houston, Texas.
Plasma concentrations of loratadine, DCL, and ketoconazole or cimetidine were used to determine the pharmacokinetic parameters using model-independent methods [14]. The maximum plasma concentration (Cmax) and the time of maximum concentration (tmax) were the observed values. The area under the plasma concentration-time curve from time zero to 24 h (AUC(0,24 h)) was calculated using the linear trapezoidal method. The trough plasma concentrations (Cmin) on days 7 through 11 were the plasma concentrations obtained prior to each 07.00 h dose or at 24 h following the last 07.00 h dose (Cmin 11).
For the cimetidine study, all sampling times on day 10 were relative to the 07.00 h dose of loratadine (Treatments D and E) or placebo (Treatment F). Since cimetidine was administered at 09.00 h, the AUC(0,24 h) for cimetidine on day 10 included the 22–24 h interval from the previous 09.00 h dose (on day 9) instead of the 22–24 h interval on day 10. Since steady-state had been reached by day 10, the AUCs in question should be equivalent to each other, i.e. AUC(22,24 h) day 9 = AUC(22,24 h) day 10.
Safety assessments
The primary endpoint was the difference between the baseline and day 10 mean QTc intervals obtained via electrocardiogram (ECG). The safety and tolerability of the treatments were evaluated by physical examinations, ECGs and routine clinical laboratory tests performed prior to and at the conclusion of the study. Additional ECGs were performed for each treatment period on day 0 at approximately 07.00 h, 08.00 h, 09.00 h, 10.00 h, 11.00 h, 13.00 h, 15.00 h, 19.00 h, and 23.00 h, daily during treatment (approximately 2 h after the 07.00 h dose), and on day 10 prior to the 07.00 h dose and at 1, 2, 3, 4, 6, 8, 12, 16, 24 and 48 h after the 07.00 h dose. A total of 88 ECGs were performed for each volunteer throughout the study. All ECGs were performed after at least 3 min in the supine position as a 12-lead ECG recorded at 25 mm s−1 and reporting ventricular rate and PR, QRS, QT and QTc intervals as well as a 2 min rhythm strip recorded at 50 mm s−1 and reporting rhythm lead group I, V2, and aVF using an automated Marquette electrocardiograph (Marquette Electronics, Inc., Milwaukee, WI). Each day 0 and day 10 automated ECG was read by the Principal Investigator to verify the automated report and to insure accuracy. ECG results were closely monitored throughout the study. Based on ECG morphology, blood samples were to be obtained, when warranted, for determination of serum magnesium and potassium concentrations.
If any QTc interval measurement (calculated automatically by the Marquette Mac II electrocardiograph using Bazett's formula) increased to greater than 500 ms, or if any ECG finding, in the Principal Investigator's opinion, precluded further treatment, the volunteer did not receive any further study treatments during that period. During the course of the study, no QTc interval was observed greater than 436 ms, and no ECG finding precluded further treatment.
Laboratory tests were repeated prior to initial treatment administration and 48 h after the morning dosage administration on day 10 of each treatment period. Vital signs after 3 min sitting were recorded at screening, pretreatment (day 0), and on each treatment day prior to, and 30 min after each 07.00 h treatment administration. Thereafter, vital signs were recorded at 24 and 48 h after the last 07.00 h dose of each treatment period. Throughout the study, volunteers were continually observed and questioned for adverse events.
Statistical methods
The primary pharmacokinetic parameters were log-transformed AUC(0,24 h) and Cmax. The change from the baseline (day 0) maximum QTc to the day 10 maximum QTc was the primary pharmacodynamic parameter. The pharmacokinetic and pharmacodynamic parameters were analysed using an analysis of variance (anova) model extracting the effects due to subject, period and treatment. Any subject missing a parameter in either period was excluded from the analysis of that parameter.
The 90% confidence intervals (90%CI) for the mean difference between the two treatments and the power to detect a 20% difference in treatment means for an alpha level of 0.05 (two-tailed) were calculated for the primary pharmacokinetic parameters and original-scale AUC, Cmax andCmin using the pooled residual error and associated degrees of freedom from the anova. For the primary pharmacodynamic parameter, QTc, the 95% confidence intervals (95%CI) were calculated for the mean difference between each pair of treatments using the pooled residual error and associated degrees of freedom from the anova.
The pharmacokinetic parameters were examined for extreme values by reviewing the studentized ranges of deviations from the expected value derived from the anova to see if any value exceeded 3.
Results
Study conduct
All 24 volunteers in the cimetidine trial completed the protocol. Twenty-four of 25 volunteers completed the ketoconazole trial; one volunteer withdrew for personal reasons following the first period of loratadine administration. He reported no adverse events and exhibited no clinically relevant ECG abnormalities. The plasma drug concentrations of the replacement subject were not consistent with the treatments assigned to him by the random code. Because it was indeterminable whether the error was made during treatment administration or during the labelling of the plasma samples, the treatments received could not be determined with certainty, and therefore the data from this volunteer were excluded from the statistical and pharmacokinetic analyses performed for this study.
Pharmacokinetic and statistical analysis
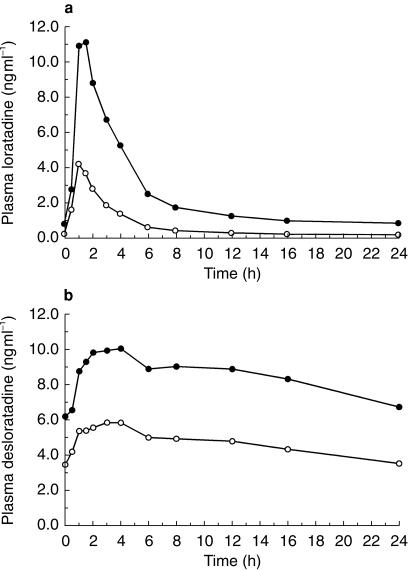
The mean pharmacokinetic parameters on day 10 are shown in Tables 1a and 1b. Steady-state was reached for all study medications by day 10. Compared with administration of loratadine alone, concomitant administration of loratadine and ketoconazole increased mean loratadine Cmax from 4.64 to 12.6 ng ml−1 and AUC(0,24 h) from 16.6 to 57.6 ng ml−1 h, and also increased mean DCL Cmax from 6.63 to 11.7 ng ml−1 and AUC(0,24 h) from 112 to 204 ng ml−1 h. Mean plasma concentration-time curves on day 10 for the ketoconazole study are shown in Figure 1a for loratadine and Figure 1b for DCL.
Table 1a.
Mean pharmacokinetic parameters on day 10 following treatment with loratadine, ketoconazole, or loratadine plus ketoconazole.
| Treatment | Analyte | Cmax* (mean,%CV) | tmax (h) (mean,%CV) | AUC(0,24h)† (mean,%CV) |
|---|---|---|---|---|
| Loratadine | Loratadine | 4.64 (75) | 1.20 (27) | 16.6 (77) |
| Desloratadine | 6.63 (83) | 2.96 (102) | 112 (130) | |
| Ketoconazole | Ketoconazole | 5.32 (39) | 3.80 (151) | 53.9 (51) |
| Loratadine plus ketoconazole | Loratadine | 12.6 (45) | 1.50 (53) | 57.6 (62) |
| Desloratadine | 11.7 (89) | 5.0 (88) | 204 (113) | |
| Ketoconazole | 5.11 (42) | 2.70 (157) | 51.8 (52) |
ng ml −1 for loratadine and desloratadine, µg ml−1 for ketoconazole.
ng ml −1 h for loratadine and desloratadine, µg ml−1 h for ketoconazole.
Table 1b.
Mean pharmacokinetic parameters on day 10 following treatment with loratadine, cimetidine, or loratadine plus cimetidine.
| Treatment | Analyte | Cmax* (mean,%CV) | tmax (h) (mean,%CV) | AUC(0,24h) † (mean,%CV) |
|---|---|---|---|---|
| Loratadine | Loratadine | 4.73 (119) | 1.56 (66) | 24.1 (157) |
| Desloratadine | 5.25 (87) | 3.00 (77) | 83.0 (117) | |
| Cimetidine | Cimetidine | 1.76 (30) | 7.83 (45) | 19.2 (23) |
| Loratadine plus cimetidine | Loratadine | 8.14 (77) | 1.63 (49) | 40.9 (135) |
| Desloratadine | 5.55 (87) | 3.31 (80) | 88.2 (29) | |
| Cimetidine | 1.64 (39) | 7.83 (43) | 18.1 (29) |
ng ml −1 for loratadine and desloratadine, µg ml−1 for cimetidine.
ng ml −1 h for loratadine and desloratadine, µg ml−1 h for cimetidine.
Figure 1.
Mean plasma concentrations of loratadine or desloratadine (DCL) vs time at day 10 steady state in healthy male volunteers taking loratadine alone (○) or in combination (•) with ketoconazole. (a) Loratadine concentrations (ng ml−1) vs time (h) after dosing. (b) DCL concentrations (ng ml−1) vs time (h) after dosing.
Plasma concentrations of ketoconazole on day 10 were similar following treatments with loratadine plus ketoconazole and ketoconazole alone. Concomitant administration of loratadine and ketoconazole gave similar mean ketoconazole Cmax (5.11 vs 5.32 µg ml−1) and AUC(0, 24 h) (51.8 vs 53.9 µg ml−1 h) compared with those obtained following administration of ketoconazole alone.
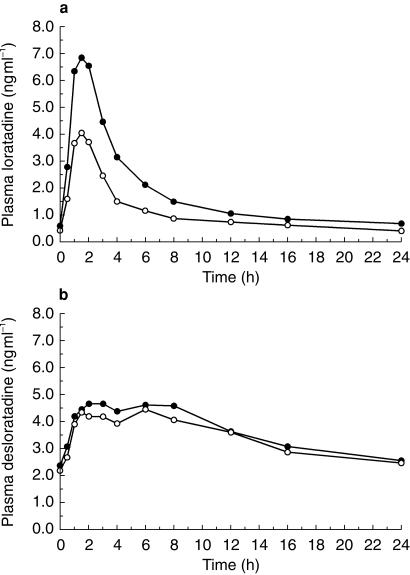
In the cimetidine study, mean plasma concentrations of loratadine on day 10 following treatment with loratadine plus cimetidine were higher than those obtained following treatment with loratadine alone while plasma concentrations of DCL following both treatments were similar. Concomitant administration of loratadine and cimetidine increased mean loratadine Cmax from 4.73 to 8.14 ng ml−1 and AUC(0,24 h) from 24.1 to 40.9 ng ml−1 h and gave similar mean DCL Cmax (5.55 vs 5.25 ng ml−1) and AUC(0,24 h) (88.2 vs 83.0 ng ml−1 h). Mean plasma concentration-time curves are shown in Figure 2a for loratadine and Figure 2b for DCL.
Figure 2.
Mean plasma concentrations of loratadine or desloratadine (DCL) vs time at day 10 steady state in healthy male volunteers taking loratadine alone (○) or in combination with cimetidine (•). (a) Loratadine concentrations (ng ml−1) vs time (h) after dosing. (b) DCL concentrations (ng ml−1) vs time (h) after dosing.
Plasma concentrations of cimetidine on day 10 were similar following treatments with loratadine plus cimetidine and cimetidine alone. Concomitant administration of loratadine and cimetidine gave similar mean cimetidine Cmax (1.64 vs 1.76 µg ml−1) and AUC(0,24 h) (18.1 vs 19.2 µg ml−1 h) compared with administration of cimetidine alone.
Statistical evaluations of log-transformed data showed that concomitant administration of loratadine and ketoconazole significantly (P < 0.05) increased the loratadine Cmax by 223% (90%CI 155–309%) and AUC(0,24 h) by 307% (90%CI 215–428), and the DCL Cmax by 67% (90%CI 51–84) and AUC(0,24 h) by 73% (90%CI 62–85) compared with the administration of loratadine alone. Concomitant administration of loratadine and cimetidine significantly (P < 0.05) increased the loratadine Cmax by 121% (90%CI 177–276) and AUC(0,24 h) by 103% (90%CI 70–142) compared with administration of loratadine alone, while concomitant administration of loratadine and cimetidine had no significant effect on the DCL Cmax (P = 0.236) but significantly (P < 0.05) increased AUC(0,24 h) compared with administration of loratadine alone. However, the difference in AUC(0,24 h) was only 6% with a 90% confidence interval of 1–11%. Concomitant administration of loratadine and ketoconazole had no significant effect on the ketoconazole Cmax (P = 0.335) and AUC(0,24h) (P = 0.342) compared with administration of ketoconazole alone. Similarly concomitant administration of loratadine and cimetidine had no significant effect on the cimetidine Cmax (P = 0.222) and AUC(0,24h) (P = 0.167) compared with administration of cimetidine alone.
Electrocardiographic evaluation
As expected in a random population of young, healthy volunteers, the Marquette Mac II automated electrocardiograph recorded a variety of cardiac electrical changes. Observed ECG changes included sinus bradycardia (defined as ventricular rates of < 60 beats min−1) nonspecific T wave abnormalities, and high voltage criteria suggestive of LVH. These abnormalities were sporadic, did not follow a pattern, were not associated with any particular treatment, and fell within the limits of normal variation for young, healthy subjects. They were evaluated and considered by the Principal Investigator to be within the limits of normal variation.
Therefore, there were no clinically relevant ECG changes associated with administration of any study treatment. No volunteer had an ECG that required blood samples to be obtained for determination of serum magnesium and potassium concentrations. There were no subjects who discontinued treatment or were withdrawn from the study due to ECG changes or increases in their QTc interval.
The primary electrocardiographic repolarization parameter, the change from baseline (day 0) to day 10 maximum QTc interval, was evaluable for 23 volunteers in the ketoconazole study and 24 volunteers in the cimetidine study. In the ketoconazole study, while not statistically significant, mean QTc intervals decreased 0.3 ms, 3.9 ms and 1.6 ms in the loratadine + ketoconazole, loratadine, and ketoconazole treatment groups, respectively (Table 2a). Pairwise comparisons of treatment group means for QTc were not statistically different (P > 0.24). The difference between means for the loratadine + ketoconazole vs loratadine alone treatment groups was 3.6 ms (95%CI−2.2–9.4 ms). The difference between means for the loratadine + ketoconazole vs ketoconazole alone treatment groups was 1.3 ms (95%CI−4.4–7.1 ms). In the cimetidine study, mean QTc intervals also decreased 2.3 ms in the loratadine plus cimetidine treatment group. Mean QTc intervals for the loratadine alone and the cimetidine alone treatment groups significantly decreased 5.4 and 3.2 ms, respectively (P < 0.05) (Table 2b). Pairwise comparisons of treatment group means for QTc were not statistically different (P > 0.05). The difference between means for the loratadine + cimetidine vs loratadine alone treatment groups was 3.2 ms (95%CI−1.6–7.9 ms). The difference between means for the loratadine + cimetidine vs cimetidine alone treatment groups was 1.0 ms (95%CI−3.8–5.8 ms).
Table 2a.
Mean absolute and mean percent change from baseline in ECG parameters following treatment with loratadine, ketoconazole, or loratadine plus ketoconazole.
| Parameter | Ketoconazole | Loratadine | Loratadine plus ketoconazole |
|---|---|---|---|
| VR (Absolute change (beats min−1) (%) | 6** (9.92) | 11** (16.08) | 12** (17.55) |
| PR (Absolute change (ms) (%) | 0 (0) | −0.35 (−0.19) | 2.96 (2.18) |
| QRS (Absolute change (ms) (%) | −0.70 (−0.72) | −0.17 (−0.03) | −2.43* (−2.31) |
| QT (Absolute change (ms) (%) | −18.6** (−4.12) | −33.2** (−7.61) | −33.6** (−7.72) |
| QTc (Absolute change (ms) (%) | −1.65 (−0.36) | −3.91 (−0.92) | −0.3 (−0.03) |
p ≤0.05
p <0.01.
Table 2b.
Mean absolute and mean percent change from baseline in ECG parameters following treatment with loratadine, cimetidine, or loratadine plus cimetidine.
| Parameter | Cimetidine | Loratadine | Loratadine plus cimetidine |
|---|---|---|---|
| VR (Absolute change (beats min−1) (%) | 6.7* (10.2) | 9.4* (14.1) | 8.9* (12.5) |
| PR (Absolute change (ms) (%) | 2.9 (1.7) | −0.4 (−0.3) | −0.5 (−0.1) |
| QRS (Absolute change (ms) (%) | −0.8 (−0.8) | −2.0 (−1.9) | −2.2* (−2.2) |
| QT (Absolute change (ms) (%) | −21.5* (−4.8) | −35.0* (−7.9) | −16.5* (−3.6) |
| QTc (Absolute change (ms) (%) | −3.2* (−0.8) | −5.4* (−1.3) | −2.3 (−0.5) |
P ≤ 0.05.
QTc intervals followed the expected distribution on days 0 and 10, and no outlying values were noted for any volunteer. No QTc interval increased more than 5% following treatment administration, and none was greater than 436 ms.
Maximum QTc intervals for the volunteer in the ketoconazole study with inconsistent study assignment and pharmacologic data increased 1% following 10 days of drug administration in periods 1 and 3 and did not change in period 2. No QTc interval for this volunteer was greater than 420 ms following study drug administration in any study period.
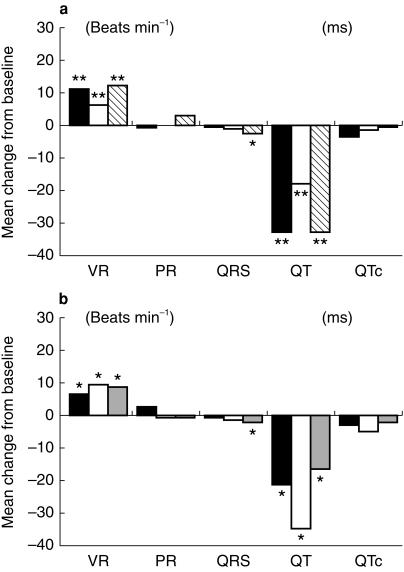
The changes in maximum PR interval, QT interval, and QRS complex measurements, and ventricular rates were also analysed in the same manner as the primary electrocardiographic parameter (see Tables 2a and 2b and Figure 3a,b). With respect to mean changes from baseline for maximum day 10 values in the ketoconazole study, statistically significant (P < 0.05) changes from baseline were observed for ventricular rate, QRS complex and QT interval. However, there were no statistically significant differences noted between the treatment groups for any ECG parameter. For the volunteer in the ketoconazole study with inconsistent study assignment and pharmacologic data, there were no significant changes in maximum PR interval, QT interval and QRS complex measurements following administration of any study treatments. Ventricular rates increased 17% following drug administration in period 1 and decreased 6% and 2.4% following in periods 2 and 3, respectively.
Figure 3.
Mean change from baseline for maximum value for electrocardiographic parameters. Absolute changes are shown on y axis, ECG parameters are shown on x axis. (a) Ketoconazole interaction study: ▪ loratidine; □ ketoconazole;  loratidine + ketoconazole. (b) Cimetidine interaction study: ▪ loratidine; □ cimetidine;
loratidine + ketoconazole. (b) Cimetidine interaction study: ▪ loratidine; □ cimetidine;  loratidine + cimetidine.
loratidine + cimetidine.
In the cimetidine study, there were no statistically significant differences in the mean changes from baseline for maximum day 10 values noted between the treatment groups for PR intervals, QRS complexes, or ventricular rates. Mean QT interval measurements decreased following 10 days of study drug administration in all treatment groups. Statistically significant differences were seen between the decreases in QT interval values for loratadine + cimetidine and the loratadine treatment groups (P < 0.01) and between the loratadine and cimetidine treatment groups (P = 0.02).
Safety evaluation
Evaluation of safety data was based on all volunteers. There were no physical examination changes noted following completion of the study. No ECG finding precluded further treatment in any study period. Vital signs were within the range observed in healthy volunteers, and no clinically relevant laboratory abnormalities were noted.
Adverse events were reported by 7 of the 25 volunteers (28%) in the ketoconazole study. Mild to moderate headache was the predominant complaint, occurring in 6 of the 7 volunteers (86%) reporting adverse events. In each case, the headache occurred during ketoconazole treatment. All headaches resolved spontaneously and did not affect study drug administration. Gastrointestinal disturbances were reported by two volunteers: one volunteer complained of mild nausea on the fifth day of ketoconazole treatment, and another volunteer experienced moderate nausea and vomiting 14.5 h after active treatment administration, and mild dyspepsia, respectively, on the fourth and fifth days of loratadine dosage administration. Dizziness was reported by one volunteer prior to receiving any study medication. Adverse events were reported by 2 of the 24 volunteers (8%) in the cimetidine study. One volunteer reported a headache on the sixth day of treatment with loratadine, and abdominal pain and nausea on the first and second days, respectively, of the loratadine plus cimetidine treatment phase. Diarrhoea was reported by another volunteer following one dose of cimetidine. All but one of the adverse events (dizziness) were considered by the investigator to be possibly related to study treatments. There was no effect of these adverse events on study drug administration, and all affected volunteers continued in the study.
Discussion
A recent analysis of adverse drug reaction forms and accompanying commentary have been quoted as evidence that loratadine is associated with serious cardiovascular events [16, 17]. However, the data from the present studies (as well as in other published reports) demonstrate that inhibitors of the drug metabolizing enzymes P450 2D6 and P450 3A4 can significantly increase plasma loratadine concentrations without evidence of QT prolongation.
The current studies investigated the effects of inhibition of two CYP450 enzymes that contribute to loratadine metabolism, P450 3A4 and P450 2D6, by ketoconazole or cimetidine, respectively, on ECG parameters. These studies demonstrated that systemic loratadine and metabolite exposure significantly increased when loratadine was combined with ketoconazole, and only loratadine exposure following cimetidine coadministration. However, no significant changes in ECG repolarization were found (P = 0.190 for loratadine + cimetidine vs loratadine alone, and P = 0.244 for loratadine + ketoconazole vs loratadine alone). Further, the plasma concentrations achieved were lower than those found following administration of 40 mg day−1 (four times the prescribed dose), which was also not associated with adverse cardiac effects [18].
Metabolically, the cytochrome P450 isozyme CYP3A4, which is inhibited by ketoconazole, appears to play a critical role in the hepatic metabolism of loratadine, while the cytochrome P450 isozyme 2D6, which is inhibited by cimetidine, appears to play a lesser role [10].
Concomitant administration of loratadine and ketoconazole increased the loratadine and DCL Cmax and AUC(0,24 h) (307% and 73% increase, respectively) compared with those obtained following administration of loratadine alone; no effect on the plasma concentrations of ketoconazole were observed. Concomitant administration of loratadine and cimetidine increased the loratadine Cmax and AUC(0,24 h) (103% increase) but did not affect the plasma concentrations of DCL (6% increase) compared with those obtained following administration of loratadine alone; no effects on the plasma concentrations of cimetidine were observed.
Although coadministration of loratadine and ketoconazole was associated with increased plasma concentrations of loratadine and DCL, and coadministration of loratadine with cimetidine was associated with increased plasma concentrations of loratadine, no clinically relevant changes in the safety profile of loratadine were observed as assessed by ECG parameters, clinical laboratory tests, vital signs, and adverse events. Additionally, cardiac repolarization was not altered, nor were other ECG parameters.
Several other lines of investigation have suggested that loratadine does not share the cardiotoxic potential of other nonsedating antihistamines, in particular terfenadine and astemizole. First, in vitro studies have demonstrated differential concentrations of suppression of potassium channels in isolated myocytes; these channels determine ventricular action potentials and are central regulators of repolarization. Suppression of any of the five subclasses of potassium channels may prolong the QTc interval; however, suppression of two of these channels, IK1 and IKr are most likely to result in cardiac dysrhythmia. At concentrations similar to those achieved clinically by coadministration of terfenadine with ketoconazole, IK1 and IKr were significantly inhibited (40% and 90%, respectively). Conversely, significant suppression of either of these channels by loratadine or its metabolite DCL did not occur until concentrations approaching 100-fold the clinically relevant plasma concentration were reached [19–21]. These data suggest that suppression of selected cardiac potassium channels by terfenadine and not by loratadine may account for the different cardiotoxic profiles of these two drugs.
Second, in animal models high concentrations of loratadine resulting from either greater than normal dosing or from competitive inhibition of cytochrome P450 metabolism did not result in any cardiac rhythm disturbances, including repolarization abnormalities. For example, Hey et al. demonstrated in anaesthetized guinea pigs that 10 mg kg−1 or 30 mg kg−1 loratadine had no effects on QTc, while terfenadine 10 mg kg−1 significantly increased the QTc and produced torsades de pointes in some animals [22].
Third, studies with human volunteers have also demonstrated the lack of cardiotoxicity associated with high plasma concentrations of loratadine or its metabolite DCL. For example 70 healthy volunteers participated in a double-blind, placebo controlled study comparing four times the recommended daily dose or 40 mg day−1 with placebo for 13 weeks. QTc changes of 10% or more occurred in 20% of placebo subjects and 12% of those receiving loratadine, but no interval greater than 440 ms was recorded in any volunteer. The authors concluded that loratadine is unlikely to significantly prolong QTc even at doses exceeding those commonly prescribed [23].
Fourth, previous studies have also demonstrated that despite higher plasma loratadine or metabolite concentrations achieved via inhibition of metabolic pathways, no ventricular dysrhythmias were generated. Brannan et al. demonstrated that competitive inhibition of P450 pathways with erythromycin increased plasma concentrations of loratadine (40% increase) or its major metabolite DCL (46% increase). However, there were no clinically relevant ECG changes associated with these increased concentrations [11]. Conversely, Honig et al. found that coadministration of terfenadine with ketoconazole, which shares the P450 3A4 metabolic pathway, resulted in accumulation of terfenadine and significant QTc prolongation [7]. Other drugs which inhibit the 3A4 pathway such as cimetidine or macrolide antibiotics can also increase terfenadine serum concentrations and prolong the QTc [3, 6].
In summary, despite increased loratadine and loratadine metabolite concentrations resulting from coadministration with ketoconazole or cimetidine, there were no clinically significant changes in cardiac repolarization parameters observed in these studies. Furthermore, the higher exposure to loratadine obtained following coadministration of loratadine and cimetidine or ketoconazole was below that previously obtained with 40 mg loratadine which was shown to be safe and well tolerated in clinical studies.
In conclusion, loratadine 10 mg tablet dosed daily did not cause QTc prolongation in healthy volunteers when coadministered for 10 days with therapeutic doses of ketoconazole or cimetidine.
Acknowledgments
We thank Mrs Elisa Sgherza and Mr Brian Flannery for overseeing the clinical conduct of the study, Mr Scott Fettner for pharmacokinetic analyses, and Ms Lucy Shneyer for statistical analyses.
References
- 1.Kamisako T, Adachi Y, Nakagawa H, Yamamoto T. Torsades de pointes associated with terfenadine in a case of liver cirrhosis and hepatocellular carcinoma. Intern Med. 1995;34:92–95. doi: 10.2169/internalmedicine.34.92. [DOI] [PubMed] [Google Scholar]
- 2.Monahan BP, Ferguson CL, Killeavy ES, et al. Torsades de pointes occurring in association with terfenadine use. JAMA. 1990;264:2788–2790. [PubMed] [Google Scholar]
- 3.von Moltke LL, Greenblatt DJ, Duan SX, et al. Inhibition of terfenadine metabolism in vitro by azole antifungal agents and by selective serotonin reuptake inhibitor antidepressants: relation to pharmacokinetic interactions in vivo[see comments] J Clin Psychopharmacol. 1996;16:104–112. doi: 10.1097/00004714-199604000-00002. [DOI] [PubMed] [Google Scholar]
- 4.Rao KA, Adlakha A, Verma-Ansil B, Meloy TD, Stanton MS. Torsades de pointes ventricular tachycardia associated with overdose of astemizole [see comments] Mayo Clin Proc. 1994;69:589–593. doi: 10.1016/s0025-6196(12)62252-6. [DOI] [PubMed] [Google Scholar]
- 5.MacConnell TJ, Stanners AJ. Torsades de pointes complicating treatment with terfenadine. Br Med J. 1991;302:1469. doi: 10.1136/bmj.302.6790.1469-b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Honig PK, Woosley RL, Zamani K, Conner DP, Cantilena Lr., Jr Changes in the pharmacokinetics and electrocardiographic pharmacodynamics of terfenadine with concomitant administration of erythromycin. Clin Pharmacol Ther. 1992;52:231–238. doi: 10.1038/clpt.1992.135. [DOI] [PubMed] [Google Scholar]
- 7.Honig PK, Wortham DC, Zamani K, et al. Terfenadine–ketoconazole interaction. Pharmacokinetic and electrocardiographic consequences [published erratum appears in JAMA 1993; 269: 2088 ][see comments]. JAMA. 1993;269:1513–1518. [PubMed] [Google Scholar]
- 8.Simons FE, Kesselman MS, Giddins NG, Pelech AN, Simons KJ. Astemizole-induced torsade de pointes. Lancet. 1988;ii:624. doi: 10.1016/s0140-6736(88)90656-3. [DOI] [PubMed] [Google Scholar]
- 9.Nightingale SL. Warnings issued on the nonsedating antihistamines terfenadine and astemizole. JAMA. 1992;268:705. [Google Scholar]
- 10.Yumibe N, Huie K, Chen KJ, Clement RP, Cayen MN. Identification of human liver cytochrome P450s involved in the microsomal metabolism of the antihistaminic drug loratadine. Int Arch Allergy Immunol. 1995;107:420. doi: 10.1159/000237063. [DOI] [PubMed] [Google Scholar]
- 11.Brannan MD, Reidenberg P, Radwanski E, et al. Loratadine administered concomitantly with erythromycin: pharmacokinetic and electrocardiographic evaluations. Clin Pharmacol Ther. 1995;58:269–278. doi: 10.1016/0009-9236(95)90243-0. [DOI] [PubMed] [Google Scholar]
- 12.Knodell RG, Browne DG, Gwozdz GP, Brian WR, Guengerich FP. Differential inhibition of individual human liver cytochromes P-450 by cimetidine. Gastroenterology. 1991;101:1680–1691. doi: 10.1016/0016-5085(91)90408-d. [DOI] [PubMed] [Google Scholar]
- 13.Michel FB, Guendon R, Guerrero AJ. [Serum IgE in patients suffering from IgA deficiency with or without atopy] Nouv Presse Med. 1976;5:1811–1814. [PubMed] [Google Scholar]
- 14.Gibaldi M, Perrier D. Pharmacokinetics. New York: Marcel Dekker, Inc.; 1982. [Google Scholar]
- 15.Johnson R, Christensen J, Lin CC. Sensitive gas-liquid chromatographic method for the determination of loratadine and its major active metabolite, descarboethoxyloratadine, in human plasma using a nitrogen-phosphorus detector. J Chromatogr B Biomed Appl. 1994;657:125–131. doi: 10.1016/0378-4347(94)80078-2. [DOI] [PubMed] [Google Scholar]
- 16.Lindquist M, Edwards IR. Risks of non-sedating antihistamines. Lancet. 1997;349:1322. doi: 10.1016/S0140-6736(97)26018-6. 10.1016/s0140-6736(97)26018-6. [DOI] [PubMed] [Google Scholar]
- 17.Clark S. Dangers of non-sedating antihistamines. Lancet. 1997;349:1268. doi: 10.1016/S0140-6736(05)62504-4. [DOI] [PubMed] [Google Scholar]
- 18.Hilbert J, Radwanski E, Weglein R, et al. Pharmacokinetics and dose proportionality of loratadine. J Clin Pharmacol. 1987;27:694–698. doi: 10.1002/j.1552-4604.1987.tb03090.x. [DOI] [PubMed] [Google Scholar]
- 19.Ko CM, Ducic I, Fan J, Shuba YM, Morad M. Suppression of mammalian K+ channel family by ebastine. J Pharmacol Exp Ther. 1997;281:233–244. [PubMed] [Google Scholar]
- 20.Ducic I, Ko CM, Shuba Y, Morad M. Comparative effects of loratadine and terfenadine on cardiac K+ channels. J Cardiovasc Pharmacol. 1997;30:42–54. doi: 10.1097/00005344-199707000-00007. [DOI] [PubMed] [Google Scholar]
- 21.Berul CI, Morad M. Regulation of potassium channels by nonsedating antihistamines. Circulation. 1995;91:2220–2225. doi: 10.1161/01.cir.91.8.2220. [DOI] [PubMed] [Google Scholar]
- 22.Hey JA, Del Prado M, Cuss FM, et al. Antihistamine activity, central nervous system and cardiovascular profiles of histamine H1 antagonists: comparative studies with loratadine, terfenadine and sedating antihistamines in guinea-pigs. Clin Exp Allergy. 1995;25:974–984. doi: 10.1111/j.1365-2222.1995.tb00400.x. [DOI] [PubMed] [Google Scholar]
- 23.Affrime MB, Lorber R, Danzig M, Cuss F, Brannan MD. Three month evaluation of electrocardiographic effects of loratadine in humans. Allergy. 1993;48(Suppl):29. [Google Scholar]