Abstract
Aims
Lamivudine (3TC, 2′-deoxy-3′-thiacytidine) requires intracellular metabolism to its active 5′-triphosphate, 3TC-5′-triphosphate (3TCTP), to inhibit the replication of hepatitis B virus (HBV). We have investigated the activation of 3TC, in the presence and absence of a range of compounds, in HepG2 cells. The intracellular levels of the endogenous competitor of 3TCTP, 2′-deoxycytidine-5′-triphosphate (dCTP), were also determined and 3TCTP/dCTP ratios calculated.
Methods
The effects of a number of compounds on 3TC (3H; 1 μM) phosphorylation were investigated by radiometric h.p.l.c. dCTP levels were determined using a template primer extension assay. 3TCTP/dCTP ratios were calculated from these results.
Results
The phosphorylation of 3TC was significantly increased in the presence of either hydroxyurea (HU), methotrexate (MTX), or fludarabine (FLU). For example, at 100 μm HU, control 3TCTP levels were increased to 361% of control, whereas at 100 μm FLU, control 3TCTP levels were increased to 155%. dCTP pools were significantly reduced in the presence of HU and FLU, at 100 μm concentrations only. However, for all the above three compounds investigated, the ratio of 3TCTP/dCTP was favourably enhanced (e.g. at 1 μm MTX, 255% of control). Neither ganciclovir (GCV), lobucavir (LCV), penciclovir (PCV), adefovir dipivoxil (ADV), nor foscarnet (FOS) had any significant effects on 3TC phosphorylation or dCTP pools.
Conclusions
These results suggest that the activity of 3TC may be potentiated when combined with one of the modulators studied. The lack of an interaction between 3TC and the other anti-HBV agents is reassuring. These in vitro studies can be used as an initial screen to examine potential interactions at the phosphorylation level.
Keywords: 3TC, dCTP, drug interactions, HepG2 cells, phosphorylation
Introduction
Chronic hepatitis B virus (HBV) infection is a major cause of life-threatening liver disease, including cirrhosis and hepatocellular carcinoma [1, 2]. HBV infection is a very serious health problem, with over 300 million individuals (5% of the world's population) infected.
HBV is a DNA virus, but it replicates using an RNA intermediate. This requires the activity of a reverse transcriptase function that is present in the HBV DNA polymerase. The multistep mechanism of HBV replication begins with reverse transcription of pregenomic HBV RNA, mediated by the reverse transcriptase activity of HBV DNA polymerase. Next, the HBV DNA polymerase exerts its DNA-dependent DNA polymerase activity to synthesize the usually incomplete positive-sense DNA strand from the negative-sense strand template [3, 4].
The development of drugs for the treatment of HBV infection can be split into two main areas. Firstly, the use of interferon α, a naturally occurring compound that can exert an antiviral effect or act as an immunomodulator [5] and secondly, the use of nucleoside analogues that function as inhibitors of viral replication [6–8]. Interferon α is currently the only approved therapy for chronic HBV. However, the combination of a poor response rate and emerging side-effects are often associated with its use [9, 10].
Lamivudine (2′-deoxy-3′-thiacytidine; 3TC) and pen-ciclovir (PCV) are among the most promising nucleoside analogues for the treatment of HBV, and are currently undergoing clinical trials. The majority of the nucleoside analogues being considered as anti-HBV therapies were originally developed to treat other viral infections. For example, 3TC, the levorotatory isomer of a cytidine analogue, is used in the treatment of human immunodeficiency virus (HIV). In contrast, PCV, and other guanosine nucleosides such as ganciclovir (GCV) and lobucavir (LCV), are used for the treatment of herpes virus infections [11, 12]. All of the nucleoside analogues inhibit HBV replication via a common mechanism. They are phosphorylated by intracellular enzymes to their active 5′-triphosphates (TPs) which then inhibit the HBV DNA polymerase through competition with their respective endogenous 2′-deoxynucleoside-5′-triphosphates (dNTPs), and by acting as chain terminators [13–15]. However, the rapid rebound of virus and the emergence of drug resistant HBV mutants observed with 3TC therapy for example [16, 17], suggests that the use of such compounds as monotherapies will be limited. Therefore, it seems likely that the future of HBV therapy will involve the use of combination regimens, in a similar fashion to the current treatment of HIV infection.
The possibility of the use of combination therapy for the treatment of HBV infection may give rise to a number of problems. These include cross-resistance, overlapping toxicity profiles, and the potential for drug–drug interactions. The reliance on the formation of the active TPs stresses the importance of assessing drug interactions between different combinations of nucleoside analogues at the phosphorylation level.
The present study describes the determination of 3TC phosphates and 2′-deoxycytidine-5′-triphosphate (dCTP) pools in an hepatic cell line (HepG2). The ability of other anti-HBV agents (GCV, LCV, PCV, adefovir dipivoxil (ADV), foscarnet (FOS)) to alter these levels was investigated, and also a number of compounds that may be able to act as modulators (e.g. hydroxyurea (HU)). As inhibition of HBV replication by 3TC is partly dependent on competition between 3TCTP and dCTP, alteration to these levels and the subsequent 3TCTP/dCTP ratio in the presence of such compounds may result in a change in efficacy of 3TC. HepG2 cells are an immortal cell line derived from human hepatocytes and have previously been shown to be an excellent in vitro model for drug metabolism studies. HepG2 cells can also be transfected with HBV (2.2.15 cells), in order to assess the antiviral potency of these compounds [8, 15, 18–21].
Methods
Chemicals
HepG2 cells were obtained from Porton Down, Salisbury, U.K. Foetal calf serum and trypsin were purchased from Gibco Life Technologies Ltd, Paisley, Scotland, U.K. Penicillin/streptomycin and l-glutamine were acquired from Northumbria Biologicals, U.K.[2′,8′-3H] 2′-de-oxyadenosine-5′-triphosphate (dATP) (specific activity, 15–18 Ci mmol−1) was purchased from Moravek Bio-chemicals Inc., Brea, CA, U.S.A. Sequenase 2.0 enzyme was obtained from Amersham Life Science, Inc., Ohio, U.S.A. The synthetic oligonucleotide primer 5′-TTGTTGTTGTTGTTGGGCGGTGGAGGCGGC-CGCCTCCACCGCC-3′ was acquired from MWG-Biotech GmbH, Germany, or Pharmacia, U.K. [3H]-3TC, 3TC and 3TCTP were kindly donated by Glaxo-Wellcome, U.K. GCV was donated by Syntex Pharmaceuticals Ltd, Maidenhead, U.K. LCV was a gift from Bristol-Myers Squibb, Wallingford, CT, U.S.A. PCV was kindly donated by Smith Kline Beecham, U.K. ADV was a gift from Gilead Sciences, Foster City, CA, U.S.A. DE81 filter papers (25 mm DEAE paper) were purchased from Whatman, U.K. Liquid scintillation fluids (Flo-scint IV and Aqualuma Plus) were obtained from Packard, CT, U.S.A. All other drugs and chemicals were purchased from Sigma Chemical Company Ltd, U.K.
HepG2 cell maintenance
HepG2 cells were routinely maintained in 75 cm2 flasks (2–8 × 106 cells/flask), containing Basal growth media (Basal media supplemented with foetal calf serum (10%), l-glutamine (2 mm) and penicillin (5000 units ml)/streptomycin (5000 μg ml−1)) at 37°C in a humidified, 5% CO2 gassed incubator. HepG2 cells are an attached cell line requiring trypsinization in order to remove cells from the flask surface. Firstly, the existing media was poured off and discarded, and the resulting monolayer of cells was washed with Versene (PBS plus 0.25% EDTA; 3 ml). Trypsin (0.5% solution; 3 ml) was then added, and discarded after 1min. The cells were suspended in fresh Basal growth media and maintained as required, then allowing 3 h for reattachment to occur. The HepG2 cells doubled approximately every 48 h.
HepG2 cell incubations with[3H]-3TC
HepG2 cells (6–8 × 106 cells/flask) were incubated with [3H]-3TC (0.65μCi; 1 μm) in a final volume of 6 ml Basal growth media. Cells were incubated at 37°C for 24 h in a humidified, 5% CO2 gassed incubator. The effects of HU, methotrexate (MTX), fludarabine (FLU), GCV, LCV, PCV, ADV, and FOS on 3TC phosphorylation were investigated over therapeutically relevant concentrations. That is, they encompassed or were similar to the plasma concentrations of these compounds observed in vivo [22–28]. For example, HU was assessed by the addition of 60 μl of 1 mm and 10 mm solutions to each flask to give final concentrations of 10 and 100 μm, respectively. FLU and FOS were also studied at 10 and 100 μm. MTX was investigated at 0.1 and 1 μm. Finally, GCV, LCV, PCV, and ADV were assessed at 1 and 10 μm concentrations. Experiments were performed in duplicate on 4 separate occasions.
Cell collection, extraction and h.p.l.c. analysis
Following incubations, cells were trypsinized as described earlier and suspensions were transferred to 20 ml sterilin tubes, centrifuged (2772 g, 4min, 4°C) and the supernatant fraction discarded. The resulting cell pellets were washed with 5 ml PBS, re-centrifuged (2772 g, 4min, 4°C) and the supernatant fraction removed. Methanol (60%, 1 ml) was added to the cells, which were then vortexed and extracted overnight at 4°C. After extraction, samples were centrifuged and the methanol extracts of each set of flasks pooled. A 50 μl aliquot of the extract was counted in scintillant (4 ml) in a liquid scintillation counter to determine the total intracellular radioactivity. The 60% methanol was then evaporated under a steam of nitrogen. Samples were stored at −20°C until h.p.l.c. analysis.
Cell extracts were reconstituted in 50 μl of ddH2O and 3TC and its phosphate metabolites were identified by radiometric h.p.l.c. using a protocol previously described [29, 30].
HepG2 cell incubations and dCTP determination
Incubations were performed using identical conditions as described previously, except 3TC was nonradiolabelled. All of the incubations were performed in duplicate on four separate occasions.
Following incubations, HepG2 cells were trypsinized as described earlier and aliquots from the resulting cell suspensions (1–1.5 × 106 cells) were transfered to 1.5 ml microcentrifuge tubes. The tubes were extracted using perchloric acid and the acid extracts neutralized by the addition of freshly prepared 0.5n trioctylamine (27%) in 1′,1′,2′-trichlorotrifluoroethane (73%) (100 μl). Extracts were stored at −20°C until determination of dCTP pools by a similar procedure to that described previously [31]. Reaction mixtures (100 μl) contained 0.2μCi [3H]-dATP, 0.25 μm synthetic oligonucleotide primer, 10 mm MgCl2, 5 mm dithiothreitol, 50 mm Tris-HCl (pH 7.5) and 1 unit Sequenase 2.0 enzyme.
The reactions were performed at 37°C for 30 min (the time period to allow optimal incorporation of dNTPs). Duplicate aliquots from each reaction mixture were spotted onto Whatman DE81 paper circles (25 mm DEAE paper), which binds oligonucleotides. After drying, the paper circles were washed three times for 10 min each with 5% Na2HPO4 (1 × 8 ml, 2 × 4 ml) to remove unincorporated nucleotides, rinsed once with ddH2O (8 ml) and once with 95% ethanol (3 ml). The paper circles were dried and then counted in Aqualuma Plus scintillant (4 ml).
Cell viability
Cell viability was assessed by the tetrazolium MTT (3,(4,5-dimethylthiazole-2-yl)-2,5-diphenyltetrazolium bromide; MTT) assay. The MTT assay is based on the reduction of the soluble pale yellow tetrazolium salt to an insoluble blue formazan product by the mitochondria of cells [32, 33]. HepG2 cells were incubated with either HU, MTX, FLU, GCV, LCV, PCV, ADV, or FOS at the concentrations previously investigated in 96 well micro-titre plates (1 × 105 HepG2 cells/well; 100 μl volume) at 37°C in a humidified, 5% CO2 gassed incubator for 24 h. MTT (5 mg ml−1; 25 μl) was added 2 h prior to terminating the incubation by the addition of 20% SDS (w/v) in 50% dimethylformamide (v/v). The plates were then incubated for a further 20 h (37°C; 5% CO2), to lyse the cells and dissolve the formazan crystals within them, and then read at 570 nm.
Data manipulation and statistical methods
Cell counting and cell viability were also assessed at the conclusion of the incubations by the method of trypan blue exclusion, to aliquots of cells taken from each plate. The 3TC phosphorylation data and dCTP data were standardized to pmol per million viable cells. 3TCTP/dCTP were calculated using these data. Cell viability data as determined by the MTT assay were expressed as percentage change in formazan production. The data were analysed by analysis of variance (anova) followed by a modified t-test (Bonferroni).
Results
3TC phosphorylation results
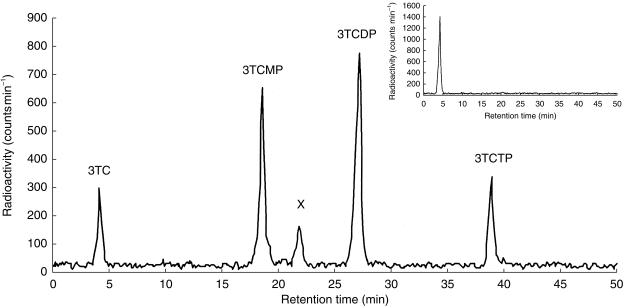
A typical h.p.l.c. profile for the detection of 3TC and phosphorylated anabolites, in HepG2 cells incubated for 24 h, is shown in Figure 1. 3TC-5′-diphosphate (3TCDP) was the predominant metabolite, accounting for about 45% of the 3TC metabolites detected. 3TC-5′-monophosphate (3TCMP) and 3TCTP accounted for approximately 32% and 15% of the metabolites, respectively. The remaining 8% of the 3TC metabolites detected corresponded to a peak between 3TCMP and 3TCDP, which was labelled X. Complete hydrolysis of all the metabolites was achieved by the addition of acid phosphatase (0.5 mg ml−1) to the extracts (Figure 1 inset). Assuming that the volume of a eukaryotic cell such as HepG2 is 1 pl [34], the intracellular concentration of 3TCTP from control cells ranged from 0.12 to 2.83 μm.
Figure 1.
Typical radiochromatogram showing the intracellular phosphorylation of [3H]-3TC (0.65μCi; 1 μm) in HepG2 cells incuhated for 24 h. Inset: radiochromatogram shows HepG2 sample after treatment with acid phosphatase (0.5mg ml−1, 37°C, 2 h). Metabolite X is postulated to be 3TCDP choline.
The effects of HU, MTX, and FLU on 3TC phosphorylation are shown in Table 1. There was a significant increase in 3TC phosphates by HU at a concentration of 100 μm (e.g. 361% of control 3TCTP production, P<0.01). MTX significantly enhanced the phosphorylation of 3TC at concentrations of 0.1 μm (e.g. 196% of control total phosphates; P<0.05), and 1 μm (e.g. 267% of control 3TCTP production; P<0.01). 3TC phosphorylation was also significantly increased in the presence of FLU at a concentration of 100 μm (155% of control 3TCTP production; P<0.05, and 193% of control total phosphates; P<0.001).
Table 1.
The effect of HU, MTX and FLU on 3TC phosphate levels and dCTP pools, in HepG2 cells incubated for 24 h.
| Drug | Total 3TC phosphates (pmol/106 cells) | Amount of 3TCTP (pmol/106 cells) | Amount of dCTP (pmol/106 cells) | 3TCTP/dCTP ratio |
|---|---|---|---|---|
| HU Control | 5.02 ± 0.47 | 0.79 ± 0.53 | 4.48 ± 1.61 | 0.176 |
| HU 10 μm | 8.51±6.14 (165) | 1.08 ± 0.56 (194) | 4.04 ±1.30 (92) | 0.267 |
| HU 100 μm | 12.22 ± 5.50 (239)** | 2.11±0.89 (361)** | 3.37 ± 1.36 (74)** | 0.626 |
| MTX Control | 5.66 ± 2.06 | 0.84 ± 0.34 | 4.03 ± 0.93 | 0.208 |
| MTX 0.1 μm | 10.56 ± 3.37 (196)* | 1.91 ± 1.19 (219)* | 5.27 ± 2.23 (128) | 0.362 |
| MTX 1 μm | 14.46 ± 5.81 (258)** | 2.45 ± 1.45 (267)** | 4.62 ± 1.68 (113) | 0.530 |
| FLU Control | 4.61 ± 0.19 | 1.11 ± 0.02 | 4.91 ± 0.47 | 0.226 |
| FLU 10 μm | 4.98 ± 0.84 (106) | 1.10 ± 0.29 (99) | 4.95 ± 0.45 (102) | 0.222 |
| FLU 100 μm | 9.03 ± 1.36 (193)*** | 1.72 ± 0.46 (155)* | 2.97 ± 0.55 (61)*** | 0.579 |
3TC concentration was 1 μm. Data expressed as mean ± s.d. (n = 4).% of control values in brackets. Data analysed by anova and modified t-test (Bonferroni).
P<0.05
P<0.01
P<0.001.
Neither GCV, LCV, PCV, ADV, or FOS significantly altered the phosphorylation of 3TC at any of the concentration studied (Tables 2 and 3).
Table 2.
The effect of GCV, LCV and PCV on 3TC phosphate levels and dCTP pools, in HepG2 cells incubated for 24 h.
| Drug | Total 3TC phosphates (pmol/106 cells) | Amount of 3TCTP (pmol/106 cells) | Amount of dCTP (pmol/106 cells) | 3TCTP/dCTP ratio |
|---|---|---|---|---|
| GCV Control | 5.98 ± 1.35 | 0.52 ± 0.45 | 5.01±0.55 | 0.104 |
| GCV 1 μm | 5.95 ± 1.05 (102) | 0.52 ± 0.49 (96) | 5.04 ± 0.63 (101) | 0.103 |
| GCV 10 μm | 5.42 ± 1.27 (91) | 0.44 ± 0.39 (90) | 5.15 ± 0.36 (103) | 0.085 |
| LCV Control | 10.66 ± 1.54 | 2.31±0.51 | 4.23 ± 0.26 | 0.546 |
| LCV 1 μm | 10.42 ± 1.66 (98) | 2.50 ± 0.82 (107) | 4.06 ± 0.95 (95) | 0.616 |
| LCV 10 μm | 10.17 ± 2.02 (97) | 2.30 ± 1.05 (99) | 4.69 ± 0.21 (111) | 0.490 |
| PCV Control | 9.68 ± 0.27 | 1.62 ± 0.33 | 4.68 ± 0.20 | 0.346 |
| PCV 1 μm | 10.04 ± 0.61 (104) | 1.83 ± 0.37 (114) | 4.64 ± 0.68 (99) | 0.394 |
| PCV 10 μm | 9.68 ± 1.04 (100) | 1.85±0.43 (120) | 5.01 ± 1.01 (107) | 0.369 |
3TC concentration was 1 μm. Data expressed as mean ± s.d. (n = 4).% of control values in brackets. Data analysed by anova and modified t-test (Bonferroni).
Table 3.
The effect of ADV and FOS on 3TC phosphate levels and dCTP pools, in HepG2 cells incubated for 24 h.
| Drug | Total 3TC phosphates (pmol/106 cells) | Amount of 3TCTP (pmol/106 cells) | Amount of dCTP (pmol/106 cells) | 3TCTP/ACTP ratio |
|---|---|---|---|---|
| ADV Control | 6.93 ± 0.84 | 0.75 ± 0.13 | 2.67 ± 0.57 | 0.281 |
| ADV 1 μm | 6.67 ± 0.60 (97) | 0.83 ± 0.21 (111) | 2.78 ± 0.79 (105) | 0.299 |
| ADV 10 μm | 6.46 ± 0.81 (94) | 0.69 ± 0.08 (94) | 3.24 ± 1.12 (121) | 0.213 |
| FOS Control | 6.29 ± 0.98 | 1.35 ± 0.33 | 4.77 ± 0.25 | 0.283 |
| FOS 10 μm | 6.04 ± 0.66 (99) | 1.26 ± 0.53 (93) | 4.70 ± 0.88 (99) | 0.268 |
| FOS 100 μm | 5.74 ± 0.77 (94) | 1.21 ± 0.43 (88) | 4.57 ± 1.02 (96) | 0.265 |
3TC concentration was 1 μm. Data expressed as mean ± s.d. (n = 4).% of control values in brackets. Data analysed by anova and modified t-test (Bonferroni).
dCTP and cell viability results
The effects of all the compounds previously investigated on 3TC phosphorylation, on dCTP levels, can also be found in Tables 1, 2 and 3.
HU, at a concentration of 100 μm, significantly reduced the levels of dCTP (74% of control dCTP levels; P<0.01; Table 1). The corresponding ratio of 3TCTP/dCTP was increased at this concentration (356% of control). HU 10 μm also caused an increase in the 3TCTP/dCTP ratio (152% of control). dCTP pools were not significantly affected by MTX, at any of the concentrations studied (Table 1). However, the 3TCTP/dCTP ratio was still increased at 0.1 and 1 μm MTX (e.g. at 1 μm, 255% of control). FLU, at a concentration of 100 μm, significantly reduced dCTP levels (61% of control dCTP levels; P<0.001; Table 1). Consequently, the 3TCTP/dCTP ratio was increased at this concentration (256% of control).
The presence of GCV, LCV, PCV, ADV, or FOS had no effect on the levels of dCTP in HepG2 cells at any of the concentrations investigated (Tables 2 and 3). No alterations in the ratios of 3TCTP/dCTP were observed with these drugs.
Finally, the MTT assay was used to determine cell viability in the presence of all the compounds studied (data not shown). FLU significantly reduced formazan production at concentrations of 10 and 100 μm (e.g. at 10 μm, 94% of control formazan production; P<0.05). Formazan production was also significantly decreased in the presence of MTX at concentrations of 0.1 and 1 μm (e.g. at 1 μm, 88% of control formazan production; P<0.001). None of the remaining compounds investigated had any significant effects on formazan production at any of the concentrations investigated.
Discussion
The limitations of current therapies for the treatment of HBV infection suggest that the use of two or more drugs in combination will provide more benefits to patients in the future. Advantages of combination therapy include synergy, the targetting of different parts of the HBV lifecycle, and an action against mutants evolved or selected under monotherapy drug pressure. A number of in vitro studies have demonstrated additive to synergistic activities of combinations of FOS with purine nucleoside analogues (e.g. GCV) against both hepadnaviruses and herpes viruses [35–38]. In contrast, the combination of purine analogues with anti-HIV nucleosides (e.g. zidovudine (ZDV), didanosine (ddI)) has resulted in antagonism of activities against HIV infection [39]. However, little is known about the effects of combinations of anti-HIV nucleosides (e.g. 3TC) with purine nucleosides against HBV infection.
The aims of these studies were to investigate the intracellular phosphorylation of 3TC in HepG2 cells, and to study the effects of various compounds on this activation. As the activity of 3TC against HBV (and HIV) is dependent on formation of the active TP, we were interested in investigating whether the presence of other compounds, which may be used in combination regimens with 3TC, could alter this activation. As the level of the competing dNTP is equally as important, the effects of these compounds on dCTP pools was also studied, so that 3TCTP/dCTP ratios could be calculated. Additionally, the effects of a number of modulators were also investigated for their ability to affect these levels in HepG2 cells. The use of such compounds may potentiate the activities of existing therapies.
The phosphorylation of 3TC in HepG2 cells is similar to that observed in previous studies with U937 cells and phytohaemagglutinin (PHA) stimulated peripheral blood mononuclear cells (PBMCs) [30]. The rate-limiting step for this activation again appears to be from 3TCDP to 3TCTP, as the levels of 3TCDP were the greatest. 3TC phosphorylation studies in 2.2.15 cells (HepG2 cells transfected with HBV) performed by Rahn et al. [21], showed that 3TCDP was also the largest anabolite formed. Although 3TCTP levels were higher than those observed in the work described here, the overall activation of 3TC was similar. However, an additional metabolite (X), postulated to be 3TCDP choline, was also observed, in a similar fashion to 3TC phosphorylation in U937 cells [30]. In comparison with previous findings in U937 cells or PHA-stimulated PBMCs, the levels of 3TC phosphates were lower in HepG2 cells. This may be due to lower amounts of intracellular enzymes compared with the other cell types investigated, or possibly may also involve the longer doubling time of HepG2 cells (observed once every 48 h).
HU (100 μm), significantly increased the phosphorylation of 3TC in HepG2 cells, with significant reductions in dCTP also noted at this concentration (Table 1). The resulting 3TCTP/dCTP ratio was increased dramatically. The mechanism behind this interaction involves the production of dCTP itself. dCTP is produced from two sources, the reduction of circulating cytidine-DP (CDP) to form dCDP, which is then phosphorylated to dCTP (the de novo pathway), and, the sequential phosphorylation of dC to dCTP (the dC salvage pathway). HU inhibits ribonucleotide reductase, the enzyme responsible for the reduction of CDP to dCDP, thereby halting the de novo pathway [40]. The corresponding fall in dCTP levels (as shown by the results) leads to activation of deoxycytidine kinase (dCK), resulting in an increased phosphorylation of 3TC, which utilizes the salvage pathway for activation. However, dCTP itself should also increase, but it is the balance of the two pathways that will ultimately determine the overall effect of HU on the levels of this metabolite.
MTX gave rise to significant increases in 3TC phosphorylation, whereas the size of the dCTP pool was unaffected at this same concentration (Table 1) MTX is thought to inhibit cell growth through an inactivation of dihydrofolate reductase [41]. A loss of cell viability was observed as determined by the MTT assay. This results in a disturbance of de novo thymidylate and/or purine biosynthesis [42]. Reductions in 2′-deoxythymidine-TP (dTTP) pools are thought to lead to a removal of feedback inhibition of dCMP deaminase activity, theoretically resulting in a reduction in dCTP levels. However, no change in dCTP was observed in the presence of this compound suggesting that dCK may have been activated to compensate for the loss of dCTP. An increase in 3TC phosphorylation supports this hypothesis. Consequently, the 3TCTP/dCTP ratio was still increased, suggesting that MTX may also have the ability to potentiate the activity of 3TC.
FLU significantly increased 3TC phosphorylation as well as significantly reducing dCTP levels, at a concentration of 100 μm (Table 1), resulting in an increased 3TCTP/dCTP ratio. Similarly, studies by Rahn et al. [21] demonstrated modest increases in 3TCDP and 3TCTP using FLU 5 μm, in 2.2.15 cells. The activity of FLU has been attributed to direct and indirect effects of the active moeity of FLU, fludarabine-TP (FaraATP), on dCK. FaraATP directly activates dCK, and also indirectly activates this enzyme through inhibition of ribonucleotide reductase (in a similar fashion to HU) [43]. The observed increase in 3TC phosphorylation is therefore expected, whereas the reduction in dCTP suggests that activation of dCK is unable to overcome the initial inhibition of ribonucleotide reductase.
The use of the above modulators again demonstrates their ability to increase the 3TCTP/dCTP ratio. This may result in a potentiation of the activity of 3TC, when used in combination with one of these compounds. Although further studies would need to be performed to assess the effectiveness of such combinations, the viability of this type of approach to therapy should not be ignored, especially as salvage therapy or pre-emptive therapy for groups at high risk of evolving a mutation.
The purine nucleosides GCV, LCV, and PCV, the nucleotide ADV, and FOS had no significant effects on both 3TC phosphorylation and dCTP pools (Tables 2 and 3). The lack of any interactions observed with these compounds suggests that the activity of 3TC may be unaltered when used in combination. However, further studies would need to be performed to assess whether 3TC has the ability to alter the activation of these compounds, and also to determine the effectiveness of combinations of 3TC with the purine nucleosides, and ADV and FOS, against HBV replication.
This work has shown that 3TC is phosphorylated in HepG2 cells, in a similar fashion to the other cell types previously investigated. The advantageous interaction between 3TC and various modulators in this cell line, and the lack of an interaction with other anti-HBV agents is reassuring. These studies may have implications for the design of combination regimens that may be used in the future treatment of HBV infection.
The next stage of the work is to determine 3TCTP and dCTP levels in liver biopsy samples from HBV-infected patients on therapy, and to determine whether the in vitro data are predictive of the situation in vivo.
Acknowledgments
We acknowledge financial support from Glaxo-Wellcome, U.K.
References
- 1.Beasley RP, Hwang L-Y, Ling C-C, Chien C-S. Hepatocellular carcinoma and hepatitis B virus. Lancet. 1981;ii:1129–1133. doi: 10.1016/s0140-6736(81)90585-7. [DOI] [PubMed] [Google Scholar]
- 2.Weissberg JI, Andres LL, Smith CL. Survival in chronic hepatitis B. an analysis of 379 patients. Ann Intern Med. 1984;101:613–616. doi: 10.7326/0003-4819-101-5-613. [DOI] [PubMed] [Google Scholar]
- 3.Ganem D, Varmus HE. The molecular biology of the hepatitis B viruses. Annu Rev Biochem. 1987;56:651–693. doi: 10.1146/annurev.bi.56.070187.003251. [DOI] [PubMed] [Google Scholar]
- 4.Wang G-H, Seegar C. The reverse transcriptase of hepatitis B virus acts as a protein primer for viral DNA synthesis. Cell. 1992;71:663–670. doi: 10.1016/0092-8674(92)90599-8. [DOI] [PubMed] [Google Scholar]
- 5.Davis GL, Hoofnagle JH. Interferon in viral hepatitis: role in pathogenesis and treatment. Hepatology. 1986;6:1038–1041. doi: 10.1002/hep.1840060537. [DOI] [PubMed] [Google Scholar]
- 6.Suzuki S, Lee B, Luo W, Tovell D, Robins MJ, Tyrrell DL. Inhibition of duck hepatitis B virus replication by purine 2′,3′-dideoxynucleosides. Biochem Biophys Res Comm. 1988;156:1144–1151. doi: 10.1016/s0006-291x(88)80752-6. [DOI] [PubMed] [Google Scholar]
- 7.Yokota T, Konno K, Chonan E, et al. Comparative activities of several nucleoside analogues against duck hepatitis B virus in vitro. Antimicrob Agents Chemother. 1990;34:1326–1330. doi: 10.1128/aac.34.7.1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Doong S-L, Tsai C-H, Schinazi RF, Liotta DC, Cheng Y-C. Inhibition of the replication of hepatitis B virus in vitro by 2′,3′-dideoxy-3′-thiacytidine and related analogues. Proc Natl Acad Sci USA. 1991;88:8495–8499. doi: 10.1073/pnas.88.19.8495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carreno V, Porres JC, Mora I, et al. A controlled study of treatment with recombinant interferon alpha in chronic hepatitis B virus infection: induction and maintenence schedules. Antiviral Res. 1987;8:125–137. doi: 10.1016/0166-3542(87)90066-0. [DOI] [PubMed] [Google Scholar]
- 10.Perrillo RP, Schiff ER, Davis GL, et al. A randomized,controlled trial of interferon alfa-2b alone and after prednisone withdrawal for the treatment of chronic hepatitis B. N Engl J Med. 1990;323:295–301. doi: 10.1056/NEJM199008023230503. [DOI] [PubMed] [Google Scholar]
- 11.Pluda JM, Cooley TP, Montaner JSG, et al. A phase I/II study of 2′-deoxy-3′-thiacytidine (lamivudine) in patients with advanced human immunodeficiency virus infection. J Infect Dis. 1995;171:1438–1447. doi: 10.1093/infdis/171.6.1438. [DOI] [PubMed] [Google Scholar]
- 12.Shaw T, Locarnini SA. Hepatic purine and pyrimidine metabolism: implications for antiviral chemotherapy of viral hepatitis. Liver. 1995;15:169–184. doi: 10.1111/j.1600-0676.1995.tb00667.x. [DOI] [PubMed] [Google Scholar]
- 13.Vere Hodge RA. Famciclovir and penciclovir. The mode of action of famciclovir including its conversion to penciclovir. Antiviral Chem Chemother. 1993;4:67–84. [Google Scholar]
- 14.Severini A, Liu X-Y, Wilson JS, Tyrrell DLJ. Mechanism of inhibition of duck hepatitis B virus polymerase by (-) -β-L-2′,3′-dideoxy-3′-thiacytidine. Antimicrob Agents Chemother. 1995;39:1430–1435. doi: 10.1128/aac.39.7.1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shaw T, Mok SS, Locarnini SA. Inhibition of hepatitis B virus DNA polymerase by enantiomers of penciclovir triphosphate and metabolic basis for selective inhibition of HBV replication by penciclovir. Hepatology. 1996;24:996–1002. doi: 10.1002/hep.510240504. [DOI] [PubMed] [Google Scholar]
- 16.Grellier L, Mutimer D, Ahmed M, et al. Lamivudine prophylaxis against reinfection in liver transplatation for hepatitis B cirrhosis. Lancet. 1996;348:1212–1215. doi: 10.1016/s0140-6736(96)04444-3. [DOI] [PubMed] [Google Scholar]
- 17.Tipples GA, Ma MM, Fischer KP, Bain VG, Kneteman NM, Tyrrell DL. Mutation in HBV RNA-dependent DNA polymerase confers resistance to lamivudine in vivo. Hepatology. 1996;24:714–717. doi: 10.1002/hep.510240340. [DOI] [PubMed] [Google Scholar]
- 18.Balzarini J, Kruining J, Wedgwood O, et al. Conversion of 2′,3′-dideoxyadenosine (ddA) and 2′,3′-didehydro-2′,3′-dideoxyadenosine (d4A) to the corresponding aryloxyphosphoramidate derivatives markedly potentiates their human immunodeficiency virus and hepatitis B virus. FEBS Lett. 1997;410:324–328. doi: 10.1016/s0014-5793(97)00616-9. [DOI] [PubMed] [Google Scholar]
- 19.Ladner SK, Otto MJ, Barker CS, et al. Inducible expression of human hepatitis B virus (HBV) in stably transfected hepatoblastoma cells: a novel system for screening potential inhibitors of HBV replication. Antimicrob Agents Chemother. 1997;41:1715–1720. doi: 10.1128/aac.41.8.1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Martin LT, Faraj A, Schinazi RF, et al. Effect of stereoisomerism on the cellular pharmacology of β-enantiomers of cytidine analogs in Hep-G2 cells. Biochem Pharmacol. 1997;53:75–87. doi: 10.1016/s0006-2952(96)00653-3. [DOI] [PubMed] [Google Scholar]
- 21.Rahn JJ, Kieller DM, Tyrrell DLJ, Gati WP. Modulation of the metabolism of β-L-(-) -2′,3′-dideoxy-3′-thiacytidine by thymidine, fludarabine, and nitrobenzylthioinosine. Antimicrob Agents Chemother. 1997;41:918–923. doi: 10.1128/aac.41.5.918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Donehower RC. An overview of the clinical experience with hydroxyurea. Semin Oncol. 1992;19:11–19. [PubMed] [Google Scholar]
- 23.Balis FM, Savitch JL, Bleyer WA. Pharmacokinetics of oral methotrexate in children. Cancer Res. 1983;43:2342–2345. [PubMed] [Google Scholar]
- 24.Kemena A, Fernandez M, Bauman J, Keating M, Plunkett W. A sensitive fluorescence assay for the quantification of fludarabine and metabolism in biological fluids. Clin Chim Acta. 1991;200:95–106. doi: 10.1016/0009-8981(91)90081-m. [DOI] [PubMed] [Google Scholar]
- 25.Dieterich DT, Poles MA, Lew EA, et al. Concurrent use of ganciclovir and foscarnet to treat cytomegalovirus infection in AIDS patients. J Infect Dis. 1993;167:1184–1188. doi: 10.1093/infdis/167.5.1184. [DOI] [PubMed] [Google Scholar]
- 26.Hoggard PG, Kewn S, Barry MG, Khoo SH, Back DJ. Effects of drugs on 2′,3′-deoxy-2′,3′-didehydrothymidine phosphorylation in vitro. Antimicrob Agents Chemother. 1997;41:1231–1236. doi: 10.1128/aac.41.6.1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pue MA, Pratt SK, Fairless AJ, et al. Linear pharmacokinetics of penciclovir following administration of single oral doses of famciclovir 125, 250, 500, and 750 mg to healthy volunteers. J Antimicrob Chemother. 1994;33:119–127. doi: 10.1093/jac/33.1.119. [DOI] [PubMed] [Google Scholar]
- 28.Barditch-Crovo P, Toole J, Hendrix CW, et al. Anti-human immunodeficiency virus (HIV) activity, safety, and pharmacokinetics of adefofir dipivoxil (9-[2-(bis-pivaloyloxymethyl)-phosphonylmethoxyethyl]adenine) in HIV-infected patients. J Infect Dis. 1997;176:406–413. doi: 10.1086/514057. [DOI] [PubMed] [Google Scholar]
- 29.Veal GJ, Barry MG, Back DJ. Zalcitabine (ddC) phosphorylation and drug interactions. Antiviral Chem Chemother. 1995;6:379–384. [Google Scholar]
- 30.Kewn S, Veal GJ, Hoggard PG, Barry MG, Back DJ. Lamivudine (3TC) phosphorylation and drug interactions in vitro. Biochem Pharmacol. 1997;54:589–595. doi: 10.1016/s0006-2952(97)00189-5. [DOI] [PubMed] [Google Scholar]
- 31.Sherman PA, Fyfe JA. Enzymatic assay for deoxynucleoside triphosphates using synthetic oligonucleotides as template primers. Anal Biochem. 1989;180:222–226. doi: 10.1016/0003-2697(89)90420-x. [DOI] [PubMed] [Google Scholar]
- 32.Mosman T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytoxicity assays. J Immunol Meth. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 33.Denizot F, Lang R. Rapid colorimetric assay for cell growth and survival. Modifications to the tetrazolium dye procedure giving improved sensitivity and reliability. J Immunol Meth. 1986;89:271–277. doi: 10.1016/0022-1759(86)90368-6. [DOI] [PubMed] [Google Scholar]
- 34.Furman PA, Fyfe JA, St Clair MH, et al. Phosphorylation of 3′-azido-3′-deoxythymidine and selective interaction of the 5′-triphosphate with human immunodeficiency virus reverse transcriptase. Proc Natl Acad Sci USA. 1986;83:8333–8337. doi: 10.1073/pnas.83.21.8333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Crumpacker CS, Kowalsky PN, Oliver SA, Schnipper LE, Field AK. Resistance to herpes simplex virus to 9-[(2-hydroxy-1-(hydroxymethyl) -ethoxy) methyljguanine: physical mapping of drug synergism within the viral DNA polymerase locus. Proc Natl Acad Sci USA. 1984;81:1556–1560. doi: 10.1073/pnas.81.5.1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Freitas VR, Fraser-Smith E, Matthews T. Increased efficacy of ganciclovir in combination with foscarnet against cytomegalovirus and herpes virus type 2 in vitro and in vivo. Antiviral Res. 1989;12:205–212. doi: 10.1016/0166-3542(89)90030-2. [DOI] [PubMed] [Google Scholar]
- 37.Manishewitz JF, Quinnan GV, Lane C, Wittek AE. Synergistic effect of ganciclovir and foscarnet on cytomegalovirus replication in vitro. Antimicrob Agents Chemother. 1990;34:373–375. doi: 10.1128/aac.34.2.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Civitico G, Shaw T, Locarnini S. Interaction between ganciclovir and foscarnet as inhibitors of duck hepatitis B virus replication in vitro. Antimicrob Agents Chemother. 1996;40:1180–1185. doi: 10.1128/aac.40.5.1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Medina DJ, Hsiung GD, Mellors JW. Ganciclovir antagonizes the anti-human immunodeficiency virus type 1 activity of zidovudine and didanosine in vitro. Antimicrob Agents Chemother. 1992;36:1127–1130. doi: 10.1128/aac.36.5.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hurta RA, Wright JA. Amplification of the genes for both components of ribonucleotide reductase in hydroxyurea resistant mammalian cells. Biochem Biophys Res Comm. 1990;167:258–264. doi: 10.1016/0006-291x(90)91759-l. [DOI] [PubMed] [Google Scholar]
- 41.Burchall JJ. Comparative biochemistry of dihydrofolate reductase. Ann New York Acad Sci. 1971;186:143–152. doi: 10.1111/j.1749-6632.1971.tb46965.x. [DOI] [PubMed] [Google Scholar]
- 42.Blakley RL. In The biochemistry of folk add and related pteridines. New York: John Wiley & Sons, Inc.; 1969. [Google Scholar]
- 43.Gandhi V, Plunkett W. Modulation of arabinosylnucleoside metabolism by arabinosylnucleotides in human leukemia cells. Cancer Res. 1988;48:329–334. [PubMed] [Google Scholar]