Abstract
Aims
To evaluate whether a year long clinical pharmacy program involving development of professional relationships, nurse education on medication issues, and individualized medication reviews could change drug use, mortality and morbidity in nursing home residents.
Methods
A cluster randomised controlled trial, where an intervention home was matched to three control homes, was used to examine the effect of the clinical pharmacy intervention on resident outcomes. The study involved 905 residents in 13 intervention nursing homes and 2325 residents in 39 control nursing homes in south-east Queensland and north-east New South Wales, Australia. The outcome measures were: continuous drug use data from government prescription subsidy claims, cross-sectional drug use data on prescribed and administered medications, deaths and morbidity indices (hospitalization rates, adverse events and disability indices).
Results
This intervention resulted in a reduction in drug use with no change in morbidity indices or survival. Differences in nursing home characteristics, as defined by cluster analysis with SUDAAN®, negated intervention-related apparent significant improvements in survival. The use of benzodiazepines, nonsteroidal anti-inflammatory drugs, laxatives, histamine H2-receptor antagonists and antacids was significantly reduced in the intervention group, whereas the use of digoxin and diuretics remained similar to controls. Overall, drug use in the intervention group was reduced by 14.8% relative to the controls, equivalent to an annual prescription saving of $A64 per resident (approximately £25).
Conclusions
This intervention improved nursing home resident outcomes related to changes in drug use and drug-related expenditure. The continuing divergence in both drug use and survival at the end of the study suggests that the difference would have been more significant in a larger and longer study, and even more so using additional instruments specific for measuring outcomes related to changes in drug use.
Keywords: aged, clinical pharmacy services, cost effectiveness, drug utilization, education, long-term care, nursing homes, patient care team, patient outcome
Introduction
Elderly residents of long-term care facilities are particularly vulnerable to adverse outcomes as a consequence of inappropriate drug use [1–2]. Psychoactive drug use is a particular problem [3], especially in Australian nursing homes where use is amongst the highest in the world [4]. Drug therapy in the elderly can be optimized by careful review [5–6]. Indeed, in one study of 2000 long-stay geriatric ward patients, half of the medications used were ceased without detriment to patient well-being [8]. Pharmacist-conducted medication reviews can have a significant impact on drug use outcomes [6–8]. A number of reports have also advocated nurse education about medication use in nursing homes [5, 9]. Such education may be appropriate given nurses' influence in this area [1–10].
Few studies have used large randomised, controlled, clinical trials to evaluate resident health outcomes resulting from clinical pharmacy interventions in nursing homes. Gurwitz et al. [1] suggested that 16 previous studies were deficient in the following respects: no control group; no clinical outcome measures; inadequate use of nursing staff to influence change; and data analysis by drug use per provider rather than drug use per resident. Controlled trials of pharmacist-conducted medication reviews in US nursing homes are difficult to facilitate because such reviews are mandatory [11]. Within the Australian system, however, it was possible to conduct a study addressing these methodological concerns in an environment where a control group was available. We report here a large nursing home based study examining the hypothesis that a clinical pharmacy intervention is cost effective in affecting drug use and improving resident health outcomes.
Methods
Sampling
Following ethical approval and pilot studies conducted outside the study area, 52 nursing homes (with 3230 residents) in Queensland and New South Wales were enrolled from 116 invited homes (randomly selected from 134 eligible homes) – an acceptance rate of 45% over a 4 month period. The nursing home eligibility criteria were (1) at least 20 residents; (2) within 3 h drive from the study centre in Brisbane; (3) supply of drugs under the Australian government medication subsidy scheme – the Pharmaceutical Benefits Scheme (PBS); (4) informed consent by each home's management to participate in the study; and (5) a central record of hospitalizations, adverse events and deaths. Sample size estimates were based on mortality estimates and the Resident Classification Instrument (RCI), a validated care needs and disability instrument of 14 elements used by the Australian government to fund nursing care [12]. The introduction of more specific drug use outcome instruments was precluded by ethical issues associated with gaining informed consent from cognitively impaired residents.
Assignment
A randomization ratio of 1 (intervention): 3 (control) was used to be within our limited budget, the cost of the study being 40% less than for a 1 : 1 sample ratio with an equivalent statistical power. In the absence of a nursing home clustering effect, a change in proportion of residents in the RCI categories 1 and 2 from 35% to 29% over 12 months and a decrease in annual mortality rate from 25% to 20% is detected at 5% (two sided) significance with a power of 80% (3230 residents, 1 : 3 sample ratio). Nursing homes were matched on resident age, RCI distribution, and bed numbers into groups of four homes after baseline data collection (Table 1). Following matching, one home from each of the 13 clusters of four was drawn from a hat and independently assigned to the intervention group (13 homes; 905 residents) and the remaining three homes to a control group (39 homes; 2325 residents).
Table 1.
Baseline distribution of intervention and control group characteristics.
| % of sample | |||
|---|---|---|---|
| Characteristic | Category | Intervention | Control |
| Resident | 1 | 2.9 | 3.1 |
| Classification | 2 | 35.6 | 39.8 |
| Instrument | 3 | 41.7 | 40.5 |
| 4 | 13.6 | 11.7 | |
| 5 | 3.0 | 3.1 | |
| Age (years) | < 60 | 2.0 | 2.6 |
| 60–69 | 6.6 | 5.4 | |
| 70–79 | 21.9 | 22.3 | |
| 80–89 | 47.4 | 46.7 | |
| 90–99 | 20.7 | 21.1 | |
| ≥ 100 | 1.7 | 1.6 | |
| Nursing home size | 20–39 | 6.8 | 12.7 |
| (number of beds) | 40–59 | 21.0 | 18.8 |
| 60–79 | 23.4 | 21.5 | |
| ≥ 80 | 48.8 | 47.1 | |
Intervention strategy
Successful intervention was anticipated to require organizational change and an active involvement of participants, as clinical pharmacy activities had not previously been undertaken in Australian nursing homes. Consequently, the 12 month intervention involved three phases: introducing a new professional role to stakeholders with relationship building; nurse education; and medication review by pharmacists who had a postgraduate diploma in clinical pharmacy.
The clinical pharmacy service model introduced to each nursing home was supported with activities such as focus groups facilitated by a research nurse, written and telephone communication, and face-to-face professional contact between nursing home staff and clinical pharmacists on issues such as drug policy and specific resident problems, together with education and medication review. This was a multifaceted intervention directly targeting nursing homes. Most of the contact with GPs was indirect, using the existing relationships between nursing homes and visiting GPs. A number of focus groups and personal interviews about the project were conducted with GPs.
In intervention homes, problem-based education sessions (6–9 seminars totalling approximately 11 h per home) were provided to nurses. Sessions addressed basic geriatric pharmacology and some common problems in long-term care (depression, delirium and dementia, incontinence, falls, sleep disorders, constipation and pain). Sessions were supported by wall charts, bulletins, telephone calls and clinical pharmacy visits, averaging 26 h contact per home over the study.
Written, referenced drug regimen reviews were prepared by the clinical pharmacists for 500 individual residents selected by the nursing home staff. The reviews highlighted the potential for: (1) adverse drug effects, (2) ceasing one or more drugs, (3) adding drugs, (4) better use of specific drug therapy, particularly psychoactive drugs, (5) nondrug interventions, and (6) adverse effect and drug response monitoring. Initial reports (61% of total) were audited by a geriatrician before dissemination. Reports were placed in each resident's nursing home records, made available to the resident's GP, and discussed with nursing staff. A sample of 159 (32%) of medication reviews were followed up to record whether changes suggested in the medication reviews led to an alteration in prescribing. Records were available for follow-up for 137 residents (22 residents were lost to follow-up through death or transfer). Drugs commonly targeted in reviews and education sessions included laxatives, histamine H2-receptor antagonists (H2 antagonists), allopurinol, quinine, antibacterials, paracetamol, nonsteroidal anti-inflammatory drugs (NSAIDs) and psychoactive drugs.
Outcome assessment
The baseline data collected for each nursing home included size, staffing mix and number of GPs attending residents. Nursing home records were used to collect demographic information for each resident [12]. Resident health outcome data collected at baseline and postintervention included mortality rate for the preceding 12 months, the number of hospitalizations in the preceding 12 months, the number of residents who experienced an adverse event in the 3 months prior to data collection (recorded by incident report forms), and scores for each of the 14 elements comprising the RCI. These RCI elements included: continence needs, maintenance of skin integrity, specialized nursing procedures, management of physical aggression, verbal disruption and other challenging behaviours, amelioration of sensory deficits (vision, hearing, speech and comprehension), activities of daily living ability (mobility, toileting, washing and dressing, and eating), and facilitation of independence. For each resident, element scores were summed to provide a composite RCI score (possible range 0–104.3). Resident survival was assessed at, on average, 22 months (control: mean 22.2, 95%CI 22.06–22.26; intervention: mean 21.8, 95%CI 21.63–21.97) from the start of the intervention.
Prescription claims for 1 year prior to, and during the trial were analysed for 13 nursing home clusters. Data were obtained from government maintained databases of subsidized drugs for a cohort of 1692 individual residents in study nursing homes for whom such records existed. The purpose of the database, pharmacist reimbursement, strengthens the validity of such drug utilization data [13–14]. Items subsidized by the Australian government are limited to the Schedule of Pharmaceutical Benefits (PBS formulary) of some 3500 items, which covers almost all medications prescribed in nursing homes [15].
To validate prescription claims data, a sample of 1328 cross-sectional medication profiles were collected for eight nursing home clusters for control and intervention homes at postintervention. These profiles recorded all items with a valid order [12] on the medication chart. including prescription-only, over-the-counter and herbal preparations. If one or more doses of the item had been given in the previous 7 days, it was also classified as ‘administered’. An audit, comparing original postintervention medication data with the same data recollected up to 6 weeks later for a 6% random sample, showed an overall reproducibility of 97% (range 92–100%). Drug use was described in terms of total drugs and in subcategories. The nomenclature and subcategory definitions used were those of the World Health Organization Nordic Anatomical Therapeutic Chemical classification index (ATC) codes [16].
Statistical analysis
The nursing home was used as the unit of analysis (cluster) for all prescription claims data. The change in yearly drug use (the trial year compared to the previous 12 months) was measured using prescription claims data. The change in each intervention home was compared with mean change in drug use for its three matched control homes using the paired Student's t-test. The mean number of items per resident per home, as measured by the medication profiles, for the intervention and control groups was also compared for the cross-section sample. Robust variance estimation techniques (SUDAAN® version 7.5, Research Triangle Institute), in which the effect of clustering within nursing homes on variance is accounted for, were used in the calculation of confidence intervals and P values. Survival curves were plotted for intervention and control groups using the Kaplan-Meier method. Survival hazard ratios were computed using Cox's proportional hazard models to compare survival in intervention and control groups, with robust variance estimation used to account for the clustering effects of nursing homes on survival times (SUDAAN®). The difference between these curves was tested using the log rank statistic. For the surviving cohort, paired t-tests were used to compare the percentage change ([postintervention minus baseline]/baseline) for the annual mortality rate, the number of Hospitalizations and residents with adverse events per nursing home and the average RCI score per nursing home. Changes in the frequency distribution of residents across subcategories of a given RCI element were compared by chi-squared analyses for the intervention and control groups. A significance level of 0.05 was used unless otherwise specified.
Results
Process and drug use assessment
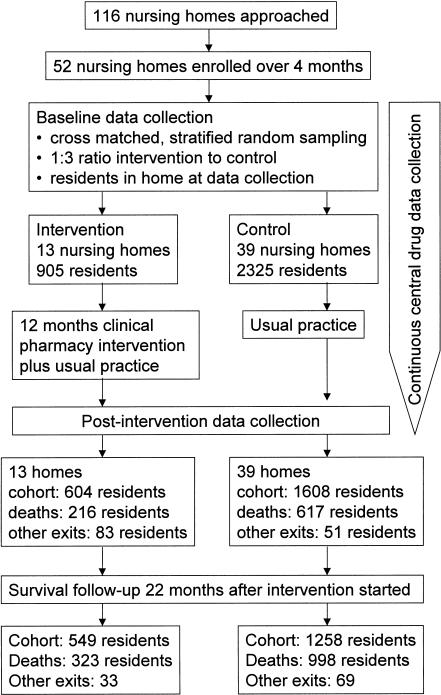
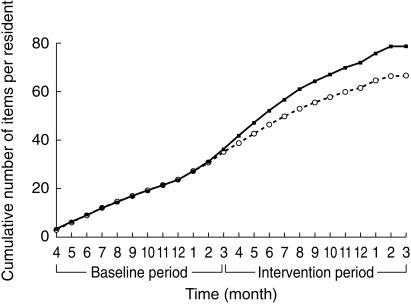
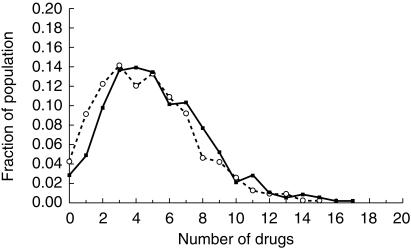
All 52 enrolled nursing homes completed the trial. Figure 1 shows the progress of the participants throughout the trial. Of the original 3230 residents, 2261 residents (70.0%) remained resident in study nursing homes at the time of postintervention data collection. Baseline use of drugs in the intervention and control groups was similar (Figure 2). In the prescription claims cohort, there was a decrease in cumulative drug use in the intervention nursing homes compared with control nursing homes (Figure 2), which just failed to reach significance for the 13 nursing home clusters (P = 0.073). The percentage change (14.8%) observed from the prescription claims data was similar to the reductions of 11.4% and 11.7% for prescribed and administered drugs in the cross-sectional sample of eight nursing home clusters (1328 residents). In this sample, there were no differences observed between the intervention (mean 6.60, 95%CI 5.60–7.60) and control (mean 7.45, 95%CI 6.98–7.92) nursing homes for the total number of drugs prescribed (P = 0.15) or between the intervention (mean 4.62, 95%CI 4.09–5.15) and control (mean 5.23, 95%CI 4.92–5.54) nursing homes for the total number of drugs administered per person (P = 0.066) with clustering. When the clustering effect of the nursing homes was not accounted for, the intervention and control residents were significantly different (P < 0.0005) for the prescription claims data and for total numbers of prescribed and administered drugs in the cross-section sample data. On average, the intervention resulted in an overall shift in the drug use by one less drug per person (Figure 3).
Figure 1.
Flow chart describing progress of residents through trial.
Figure 2.
Longitudinal evaluation of prescription claims data – Cumulative number of items dispensed per 1000 for the cohort of nursing home residents surviving to the end of the study in the intervention (○) and control groups (•).
Figure 3.
Cross-sectional evaluation of manually collected drug use data – Frequency of number of drugs administered per resident in intervention (○) and control (•) groups post intervention.
Significant reductions in medication use were also apparent for some drug categories, with trends consistent across the prescription claims (Table 2) and cross-sectional samples (Table 3), except for paracetamol. Analysis of prescription claims data showed that the supply of benzodiazepine hypnotics, NSAIDs, laxatives, H2 antagonists and antacids was significantly reduced by the intervention while digoxin and diuretics were unchanged. Allopurinol and quinine were not analysed due to the small proportion of residents taking these drugs. In the cross-sectional sample, the mean number of drugs administered/person was significantly lower for laxatives and psycholeptics (i.e. antipsychotics, anxiolytics and hypnotic-sedatives). Non-drug measures, such as pear juice as a mild laxative, were found to be effective for many residents. Post-intervention, 10.2% fewer residents were administered psychoactive medication (psycholeptics and antidepressants) and 21.3% fewer residents were administered hypnotics when compared with controls (Table 4). Paracetamol was promoted in the intervention for untreated pain and is available both on prescription and over-the-counter. The greater use of paracetamol in the intervention cross-section group (a finding not evident in the prescription data) may be explained by the paracetamol being supplied over-the-counter. The strong influence of nursing home on drug use is evident in the higher probability values obtained when the clustering effect of the nursing home is accounted for in the analysis of individual drug use (Table 3).
Table 2.
Difference between intervention homes and control homes for change in number of prescription claims items/year/1000 residents for individual drug categories.
| Change in number of prescription items/year/1000 residents (trial period – baseline period⋆) | ||||
|---|---|---|---|---|
| Drug category | Intervention | Control | Intervention-Control | P value# |
| Antacids | −228 | −146 | −82 | 0.038 |
| H2 antagonists | −267 | 18 | −285 | 0.012 |
| Laxatives | 108 | 559 | −451 | 0.008 |
| Digoxin | −96 | −84 | −12 | 0.331 |
| Diuretics | −486 | −131 | −355 | 0.494 |
| Antibacterials | −824 | −196 | −628 | 0.014 |
| NSAIDs | −489 | −250 | −239 | 0.036 |
| Paracetamol | 360 | 820 | −460 | 0.069 |
| Psycholeptics | −4447 | −2792 | −1655 | 0.059 |
| Benzodiazepines† | −597 | 278 | −875 | 0.024 |
Trial period – April to March; Baseline period – preceding 12 months.
ATC category for benzodiazepine hypnotics.
t-test.
Table 3.
Difference between intervention and controls for the postintervention cross-sectional mean number of drugs administered/person for individual drug categories.
| Analysis Mean number of drugs administered/person | |||||
|---|---|---|---|---|---|
| Drug category | Intervention | Control | Intervention-Control (95%CI)⋆ | P value⋆ | P value# |
| Antacids | 0.03 | 0.04 | −0.01 (−0.05–0.03) | 0.80 | 0.56 |
| H2 antagonists | 0.15 | 0.15 | 0.00 (−0.04–0.04) | 0.97 | 0.90 |
| Laxatives | 0.33 | 0.71 | −0.37 (−0.57—0.17) | 0.002 | < 0.0001 |
| Digoxin | 0.17 | 0.18 | −0.01 (−0.07–0.05) | 0.73 | 0.73 |
| Diuretics | 0.30 | 0.28 | 0.02 (−0.04–0.07) | 0.49 | 0.45 |
| Antibacterials | 0.10 | 0.12 | −0.02 (−0.08–0.04) | 0.57 | 0.52 |
| NSAIDs | 0.04 | 0.07 | −0.03 (−0.07–0.03) | 0.099 | 0.013 |
| Paracetamol | 0.39 | 0.33 | 0.06 (−0.04–0.16) | 0.29 | 0.021 |
| Psycholeptics | 0.59 | 0.73 | −0.14 (−0.28–0.0) | 0.044 | 0.012 |
| Benzodiazepines† | 0.20 | 0.26 | −0.06 (−0.16–0.04) | 0.29 | 0.026 |
ATC category for benzodiazepine hypnotics.
adjusted for nursing home clustering.
unadjusted for nursing home clustering.
Table 4.
Percentage of residents being administered pyschotropic medication at the end of the study.
| % of Residents | ||||
|---|---|---|---|---|
| Drug group | ATC description | Control n = 850 | Intervention n = 478 | % difference† |
| Any psychotropic | n05⋆ or n06a⋆ | 61.8 | 56.1 | 10.2 |
| Neuroleptics ‘antipsychotics per ATC’ | n05a⋆ | 29.4 | 23.2 | 24.2 |
| Psycholeptics | n05⋆ | 51.6 | 47.1 | 9.6 |
| Thioridazine | n05ac02 | 15.3 | 13.0 | 14.8 |
| Haloperidol | n05ad01 | 8.8 | 7.5 | 21.7 |
| Trifluoperazine | n05ab06 | 0.9 | 0.4 | 100 |
| Chlorpromazine | n05aa01 | 2.0 | 1.7 | 13.0 |
| Fluphenazine | n05ab02 | 0.7 | 0 | 117 |
| Pericyazine | n05ac01 | 1.2 | 0.6 | 100 |
| Pimozide | n05ag02 | 0.1 | 0 | 100 |
| Hypnotics ‘hypnotics and sedatives per ATC’ | n05c⋆ | 26.1 | 20.9 | 21.3 |
| Temazepam | n05cd07 | 22.0 | 18.2 | 19.1 |
| Nitrazepam | n05cd02 | 3.4 | 1.7 | 50.0 |
| Anxiolytics | n05b⋆ | 11.6 | 11.1 | 5.1 |
| Diazepam | n05ba01 | 6.5 | 5.2 | 21.6 |
| Oxazepam | n05ba04 | 4.9 | 6.3 | −36.8 |
| Hypnotics and/or anxiolytics | n05b⋆ or n05c⋆ | 34.8 | 30.3 | 14.2 |
| Benzodiazepines total | n05ba⋆ or n05cd⋆ | 34.2 | 29.0 | 16.6 |
| Antidepressants | n06a⋆ | 23.3 | 19.7 | 17.8 |
| Lithium and no antidepressant | n05an01 but no n06a⋆ | 0.4 | 0.4 | 0 |
Truncated ATC code.
For each specified drug or drug group: % difference = (Post-intervention percentage Control residents on drug —% intervention residents on drug × 100)/(% of residents at baseline on drug).
Outcome assessment
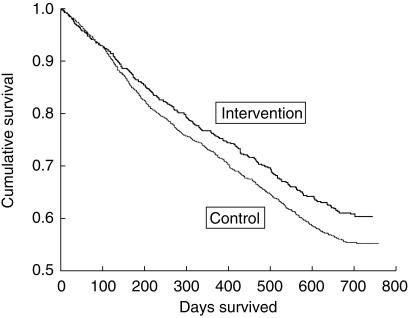
Cumulative survival in the intervention group appeared better than controls with a hazard ratio (HR) of 0.85 (Figure 4). This indicates that residents in the intervention group were 15% more likely to survive longer than those in the control group. Whilst this survival was significant when analysed in terms of individual residents (HR 0.85, 95%CI 0.75–0.96, P < 0.009), no significant differences were apparent when the nursing home clustering effect was accounted for (HR 0.85, 95%CI 0.68–1.06, P = 0.13). This compares with a Standardized Mortality Ratio [17] of 0.85 (95%CI 0.76–0.95 unclustered; 95%CI 0.69–1.05 adjusted for clustering) with adjusted mortality rates of 27.22/100 person years and 31.69/100 person years in the intervention and control groups, respectively. Table 5 shows the mean results obtained for annual mortality rates, frequency of hospitalizations, number of residents with adverse events and changes in disability index (RCI raw score) in the intervention and control groups. No significant changes were observed in annual mortality rates, frequency of hospitalizations, number of residents with adverse events or changes in the RCI disability index (raw score, elements or proportion of residents in categories 1 and 2) between intervention and control nursing home groups.
Figure 4.
Cumulative survival function curves.
Table 5.
Outcomes from 12 month medication care intervention. Pre-study and poststudy values are expressed as the mean percentage for deaths, Hospitalizations and adverse events, and mean raw score for RCI.
| Outcome measure mean (95%CI) | Study group P value | Pre-study mean (95%CI) | Post-study mean (95%CI) | % change# | |
|---|---|---|---|---|---|
| Mortality | Intervention⋆ | 28.75 (23.98–33.61) | 28.73 (22.96–34.44) | + 4.86 (−16.33–26.05) | 0.696 |
| (12 months) | Control⋆ | 33.23 (29.37–37.03) | 35.72 (31.68–39.72) | + 12.48 (−7.28–32.23) | |
| Hospitalizations | Intervention† | 17.67 (11.59–23.75) | 15.86 (10.55–21.16) | + 1.30 (−26.32–28.91) | 0.305 |
| (12 months) | Control† | 22.70 (18.83–26.57) | 18.36 (15.07–21.65) | −16.86 (−30.62–3.11) | |
| Adverse events | Intervention† | 28.74 (18.89–38.59) | 31.46 (19.86–43.06) | + 54.02 (−44.51–152.56) | 0.388 |
| (3 months) | Control† | 22.38 (16.15–28.62) | 26.00 (19.81–32.19) | + 49.07 (−21.17–119.31) | |
| RCI | Intervention† | 65.14 (61.71–68.57) | 68.55 (65.20–71.90) | + 5.52 (1.45–9.59) | 0.253 |
| (12 months) | Control† | 64.24 (60.75–67.73) | 67.83 (64.62–71.04) | + 5.73 (4.44–7.02) | |
Based on nursing home bed capacity.
In cohort of 2286 enrolled in the study and surviving until the end of the study. % difference = (#(post-pre) × 100)/pre.
A net cost saving of $A16 per resident per year was estimated by subtracting the cost of delivering the interventions (about $A48 per resident per year) from the drug cost differences between the two groups ($A64 per resident per year based on the prescription claims data). The projected net saving for a nursing home population of 74 236 in Australia is approximately $A1.2 million per year (approximately £0.47 m).
Discussion
Effect of intervention
An intervention designed to improve the quality of medication care in nursing home residents was shown to lead to a reduction in drug use without adversely affecting survival and morbidity indices. The reduction in medication use was similar (11.4–14.8%) in the cross-sectional and prescription claims analyses. Given that in the longitudinal analysis both the drug use (Figure 2) and survival (Figure 4) continue to diverge at the end of the data collection periods, the extent of the improvement in outcome should have been greater if the study had been conducted over a longer time. As limited resource availability constrained the possible number of intervention sites, a 1 : 3 (intervention:control) ratio was used to minimize type II error.
The main drug categories reduced by this intervention were laxatives and psycholeptics, the use of which is often prompted by nurses [18]. Non-drug measures, such as the use of pear juice as an alternative to laxatives, were encouraged in this study. The use of paracetamol for untreated pain was promoted in the intervention. The trend toward a higher usage in the cross-section intervention group, but not in the prescription claims group, may be accounted for by paracetamol being supplied over-the-counter (OTC). Previous work suggests psychoactive medications, laxatives and analgesics are drug categories that require medication review [18]. The lack of change in cardiovascular medicines may reflect a stronger nursing than GP response to the intervention.
The cost-effectiveness of applying this intervention to reduce drug use without adversely affecting the clinical outcomes of residents is especially important. Cost savings may be even greater in other countries because, the same drug, dosage form and strength (manufactured by the same company), can generally be purchased for less in Australia than in countries such as the UK and USA [19].
Most intervention studies have measured changes in drug use without assessing health outcomes [1]. In this study, the drug use per person for the intervention group at the end of the trial (4.6 items) reached a level similar to that reported in US studies (4.9 items) [2]. This study differed from those in the US in that it was undertaken in the absence of legislative support for pharmacy intervention in nursing homes, in a more debilitated population (control group mortality rate 25.9%, US studies 13.9% [2]) and in the presence of a national formulary (PBS).
Possible confounders
This study employed a randomised controlled trial design using nursing home as the variable to determine the sample, matching and analysis. A new practice model was introduced using an organizational change strategy emphasizing multidisciplinary involvement. Nurses requested education about medication and were positive about their involvement after the intervention, which is consistent with other reports [9–20]. While education directed toward GPs has also been shown to be effective in reducing drug use [1], this study was restricted by the large number (mean 20, median 16, range 5–56) of independent, visiting GPs per nursing home, necessitating emphasis on the nurse as the conduit for change. Indirect contact with GPs may have reduced the impact of the medication reviews although, of the 137 residents followed-up for actioning of medication reviews, 54 (39%) of residents had changes likely to be due to the medication review. Similarly, in another Australian study [21], 38% of pharmacists' recommendations made to interdisciplinary case conferences were actioned. While this acceptance rate is low compared with US studies with reported acceptance rates between 66% [23] and 85% [24], an acceptance rate of 41.6% was reported in a UK study of domiciliary medication review [22]. Since the appropriate drug use messages were included in both the medication reviews (seen by nurses and GPs) and the nurse education, medication changes cannot solely be attributed to GPs acting on medication reviews. Accordingly, significant differences were observed for drug categories most affected by nursing home culture [12]. Others have found that interdisciplinary collaboration with GPs in medication care interventions also enhances drug use outcomes [21]. In this study, the importance of the nursing home as a determinant of drug use outcomes is evidenced by the increase in confidence interval and decrease in statistical significance found for variables after accounting for the nursing home clustering effect.
The collection of outcome indices within the resource constraints of the study and issues of informed consent for cognitively impaired residents necessitated the use of indicators routinely kept by the nursing homes. The Resident Classification Instrument was a tool for resource allocation based on care needs. It has been reported to have high interrater reliability [25] and, nationally, only approximately 6–7% of classifications were downgraded in government audits of the assessments [26]. RCI scores and categories have been found to have strong correlation (Spearman's Correlation Coefficient = 0.71 and −0.70, respectively, P < 0.001) with a subset of the US Minimum Data Set Plus (MDS +), the Resource Utilization Groups Version III [25]. Australian nursing homes are required to have an incident reporting system that is ‘used and regularly monitored’ [27]. However, there was wide variation in the level of reporting between nursing homes related to the policy or philosophy of the home which could have overshadowed the effects of psychoactive drug reduction. It should be noted however, that the number of falls reported after a prevention or reduction programme has increased in several studies [28–31]. In two of these studies, the increase was attributed in part to improved reporting of falls [28–29]. Whilst generic instruments associated with everyday nursing home operation were used in this study, it is possible that instruments more specifically related to drug use outcomes may have provided clearer evidence of the extent and nature of the benefits achieved from this intervention.
The low overall enrolment rate (45%) was largely due to a time constraint imposed on the recruitment period so as to enable the study to finish on schedule with a 12 month intervention, and was comparable with that forecast (50%). It was not considered to impact significantly on the internal validity of the results as nursing homes were randomised to intervention or control after enrolment. It may, however, affect external validity in that these results may only be applicable to homes willing to participate or be involved in such an intervention when given a limited time for enrolment. As discussed earlier, the limited duration and size of the study were possible sources of type II error.
Conclusions
Clearly any cost-effective health intervention, in which outcomes (such as improved survival without morbidity increases) can be monitored, should be given serious consideration. We believe that residents of all nursing homes would benefit from an intervention of the combined education and medication review type described in the present study, including co-operation between the clinical pharmacist, the nurse and the GP in the prescribing and administration of drugs. A major outcome of this study has been the Australian Government-funded implementation of an accredited pharmacist-conducted medication review program for all Australian nursing homes.
Acknowledgments
This study was supported by the Commonwealth Government of Australia under the Pharmaceutical Education Program.
We wish to thank all members of the research team, for their contributions to the implementation and administration of this large project, the staff of the nursing homes and the visiting general practitioners. The contribution of representatives from nursing home, general practitioner, nursing, pharmacy, and consumer organizations, government, Hospital, academia and research staff to the steering and other committees was invaluable and greatly appreciated.
References
- 1.Gurwitz JH, Soumerai SB, Avorn J. Improving medication prescribing and utilization in the nursing home. J Am Geriatr Soc. 1990;38:542–552. doi: 10.1111/j.1532-5415.1990.tb02406.x. [DOI] [PubMed] [Google Scholar]
- 2.Macklin J. Issues Paper No 4 Issues in Pharmaceutical Drug Use in Australia. Canberra: AGPS Publications; 1992. National Health Strategy. [Google Scholar]
- 3.Avorn J, Dreyer D, Connelly K, Soumerai S. Use of psychoactive medication and quality of life in retirement homes. N Engl J Med. 1988;420:227. doi: 10.1056/NEJM198901263200406. [DOI] [PubMed] [Google Scholar]
- 4.Snowdon J, Vaughan R, Miller R, Burgess EE Tremlett. Psychotropic drug use in Sydney nursing homes. Med J Aust. 1995;163:70–72. doi: 10.5694/j.1326-5377.1995.tb126117.x. [DOI] [PubMed] [Google Scholar]
- 5.Gibbins FJ, Sen I, Vaz FS, Bose S. Clinical budgeting and drug management on long-stay geriatric wards. Age Ageing. 1988;17:328–332. doi: 10.1093/ageing/17.5.328. [DOI] [PubMed] [Google Scholar]
- 6.Kidder SW. Cost-benefit of pharmacist conducted drug regimen reviews. Consult Pharm. September/October 1987:294–398. [Google Scholar]
- 7.McGhan WF, Einarson TR, Sabers DL, et al. A meta-analysis of the impact of pharmacist drug regimen reviews in long-term care facilities. J Am Geriatr Soc. 1987;1:23–34. [Google Scholar]
- 8.Thompson JF, McGhan WF, Ruffalo RL, et al. Clinical pharmacist prescribing drug therapy in a geriatric setting: outcome of a trial. J Am Geriatr Soc. 1984;32:154–159. doi: 10.1111/j.1532-5415.1984.tb05858.x. [DOI] [PubMed] [Google Scholar]
- 9.Haynes J, Bradley M. Pharmaceutical services to residential homes – present and future. Br J Pharm Pract. October 1990:326–334. [Google Scholar]
- 10.Ray WA, Taylor JA, Meador KG, et al. Reducing antipsychotic drug use in nursing homes: a controlled trial of provider education. Arch Intern Med. 1993;153:713–721. [PubMed] [Google Scholar]
- 11.Conditions of Participation-pharmaceutical services. Federal Register. 1974;39:12–17. [Google Scholar]
- 12.Roberts MS, King M, Stokes JA, et al. Medication prescribing and administration in nursing homes. Age Ageing. 1998;27:385–392. [Google Scholar]
- 13.Avorn J, Soumerai SB. Use of computer-based Medicaid drug data to analyse and correct inappropriate medication use. J Med Sys. 1982;6:377–386. doi: 10.1007/BF00992880. [DOI] [PubMed] [Google Scholar]
- 14.Hurley SF, McNeil JJ, Jolley DJ, Harvey R. Linking prescription and patient-identifying data: a pilot study. Med J Aust. 1992;156:383–386. doi: 10.5694/j.1326-5377.1992.tb139839.x. [DOI] [PubMed] [Google Scholar]
- 15.Australian Institute of Health Welfare. Australia's Health 1994. Canberra: AGPS Publications; 1994. p. 142. [Google Scholar]
- 16.WHO Collaborating Centre for Drug Statistics Methodology. Anatomical Therapeutic Chemical (ATC) Classification Index. Oslo: World Health Organisation; [January 1994]. [Google Scholar]
- 17.Breslow NE, Day NE. Statistical Methods in Cancer Research. The Design and Analysis of Cohort Studies. IARC-Sci-Publications. 1982;2:69–71. [PubMed] [Google Scholar]
- 18.Avorn J, Gurwitz JH. Drug use in the nursing home. Ann Intern Med. 1995;123:195–204. doi: 10.7326/0003-4819-123-3-199508010-00007. [DOI] [PubMed] [Google Scholar]
- 19.Wertheimer AI, Grumer SK. Overview of international pharmacy pricing. Pharmacoeconom. 1992;2:449–455. doi: 10.2165/00019053-199202060-00005. [DOI] [PubMed] [Google Scholar]
- 20.Schmidt IK, Claesson CB, Westerholm B, Nilsson LG. Physician and staff assessments of drug interventions and outcomes in Swedish nursing homes. Ann Pharmacother. 1998;32:27–32. doi: 10.1177/106002809803200102. [DOI] [PubMed] [Google Scholar]
- 21.King MA, Roberts MS. Progress In Practice, United Kingdom Clinical Pharmacy Association Residential Spring Symposium. Leeds: [May 2000]. Multi-Disciplinary Case Conference Reviews: Improving Outcomes for Nursing Home Residents, Carers and Health Professionals; pp. 20–21. [Google Scholar]
- 22.Brackley K. What impact can community pharmacists have on medication use in the elderly? An evaluation of domiciliary based medication review by community pharmacists. Pharm World Sci. 1998;20:A4. [Google Scholar]
- 23.Vlasses PH, Lucarotti RL, Miller DA, et al. Drug therapy review in a skilled nursing facility: an innovative approach. J Am Pharm Ass. 1977;NS17:92–94. doi: 10.1016/s0003-0465(16)34242-2. [DOI] [PubMed] [Google Scholar]
- 24.Brown C. Physicians acceptance of drug-therapy recommendations using a written data and communications form. Consult Pharm. 1991;6:729–734. [Google Scholar]
- 25.Zlobicki MT, Kellett U, Scarlett S, Barnard A. Nursing home resident classification pilot study. MDS+/RUG III and RCI trials in Queensland. Proc Annu Conf Aust Ass Gerontol. 1993;28:49–53. [Google Scholar]
- 26.Commonwealth Department of Health and Family Services. Annual Report 1995–96. Canberra: Australian Government Publishing Service; 1996. p. 139. [Google Scholar]
- 27.Commonwealth Department of Health and Aged Care Aged and Community Care Division. Standards and Guidelines for Residential Aged Care Services Manual May 1998. Canberra: AGPS. Publishing; 1998. Available at: URL. http://http://www.health.gov.au/acc/manuals/sgr/contents/std-2.htm #2.11. [Google Scholar]
- 28.Taylor J, Morris M. Education, staff knowledge and falling in hostel residents. Physiother Singapore. 1999;2:128–134. [Google Scholar]
- 29.Uden G, Ehnfors M, Sjostrom K. Risk assessment and recording as the main nursing interventions in identifying risks and preventing fall injuries. J Adv Nurs. 1999;29:145–152. doi: 10.1046/j.1365-2648.1999.00874.x. [DOI] [PubMed] [Google Scholar]
- 30.Llwellyn J, Martin B, Shekleton M, Firlit S. Analysis of falls in the acute surgical and cardiovascular surgical patient. Appl Nurs Res. 1988;1:116–121. doi: 10.1016/s0897-1897(88)80021-1. [DOI] [PubMed] [Google Scholar]
- 31.Ejaz FK, Jones JA, Rose MS. Falls among nursing home residents: an examination of incident reports before and after restraint reduction programs. J Am Geriatr Soc. 1994;42:960–964. doi: 10.1111/j.1532-5415.1994.tb06587.x. [DOI] [PubMed] [Google Scholar]