Abstract
Aims
To test in vitro the constrictor and relaxation responsiveness of variously diseased segments of human saphenous vein from patients with varicose vein disease.
Methods
The vein segments were derived (i) from the inguinal saphenous vein (valvularly incompetent and slightly dilated; tissue A); (ii) from the distal end of the lower leg just above the medial ankle (competent; tissue B); (iii) from a tributary to the long saphenous vein just below the knee (dilated, incompetent and overtly varicose; tissue C). The contractile responses were tested with phenylephrine (an α-adrenergic receptor agonist) and aescin, a clinically used phlebotonic drug derived from horse chestnut extract. Relaxant responses were tested with acetylcholine and sodium nitroprusside.
Results
Both contractile agents contracted vein segments from the inguinal and ankle area with similar potency and efficacy, but were virtually without effect in the overtly varicose segments from the calf. EC50 values (molar concentration of the agonist that produces 50% of the maximum effect) in tissues A and B were 2.9 ± 0.3 and 2.5 ± 0.5 µmol l−1 (phenylephrine) and 9.4 ± 1.0 and 15.9 ± 2.5 µmol l−1 (aescin); the corresponding maximum effects (maximum effect, percent of KCl-induced contraction) were 76 ± 3 and 70 ± 4% (phenylephrine) and 70 ± 2 and 71 ± 3% (aescin) (P = NS in both cases for A vs B). In tissue C, the maximum effects were 5 ± 5% (phenylephrine) and 10 ± 7% (aescin) of KCl-induced contraction (not significantly different from zero). Acetylcholine-induced relaxation was similar for vein segments from locations A and B, whereas sodium nitroprusside was more effective in tissue B than A.
Conclusions
These findings support the notion that abnormalities within the venous wall affect venous smooth muscle contractility. Since competent and incompetent clinically normal vessels show normal contractile responses, whereas varicose vessels are not responsive to vasoactive drugs, it is likely that pharmacological treatment regimens are effective in early, but not in late stages of the disease.
Keywords: pharmacotherapy, primary varicosity, smooth muscle contractility
Introduction
Varicose veins of the lower limb affect a sizable fraction of the adult population and result in considerable suffering and huge losses to the economy. Visible varicose veins are found in 20–25% of female and 10–15% of male adults, with a high familial incidence [1]. Dilated veins and incompetent valves are the common manifestation of the disease. The consequence of the resultant reflux is a high venous pressure and venous hypertensive microangiopathy. Phleboedema, induration of the tissue in the distal leg, thrombophlebitis and ulcerations are common complications in untreated varicosity [2]. Acute manifestations, such as deep venous thrombosis and pulmonary embolism [3] are serious complications, and the chronic form of the disease results in high socioeconomic costs due to long hospitalization and work disability [4].
The aetiology of primary varicosity is still unknown. A recent theory suggests that a defective control of venous tone leads to dilatation and subsequent insufficiency. This notion is supported by clinical observations and experimental studies in which varicose veins (or segments thereof) showed a reduced ability to contract in response to several vasoconstrictors including α-adrenoceptor agonists and endothelin [5–7]. The possible role of an imbalance between endothelial derived contracting and relaxing factors was reported by our group [8]. Several drugs including flavonoids and horse chestnut seed extract (aescin) are currently used for short-term treatment of varicose vein disease, but whether they raise venous tone as part of their mechanism of action is controversial [9–10].
In the present study we determined the in vitro reactivity of long saphenous vein derived from patients with varicose vein disease. We hypothesized that the efficacy of drugs raising venous tone may depend on the severity of the disease as evident from clinical symptoms. Hence, we obtained vein segments from three anatomical locations of the same patient, i.e. from the inguinal saphenous vein (valvularly incompetent and clinically nonvaricose tissue), the distal portion of the long saphenous vein just above the medial ankle (competent), and from a tributary to the long saphenous vein just below the knee (incompetent, dilated and overtly varicose tissue), and tested their reactivity to two contractile agents, phenylephrine and aescin, and two relaxant drugs, acetylcholine and sodium nitroprusside.
Methods
Patients and test groups
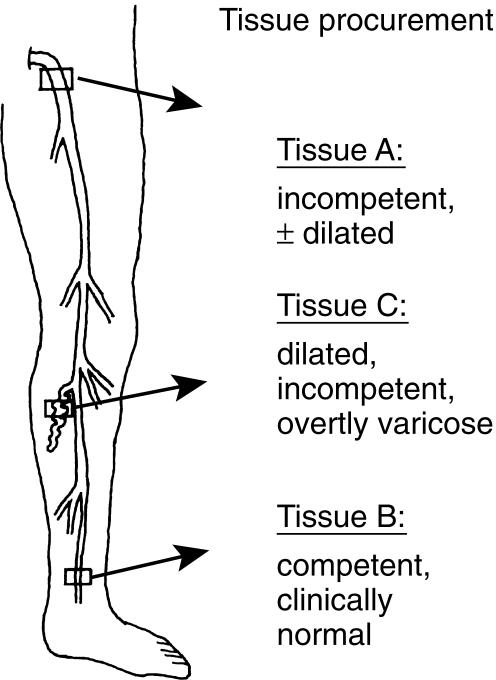
Tissues were obtained from seven female patients (mean age, 46 years) with primary varicosity, hospitalized for surgical vein stripping. All patients received general anaesthesia and consented to the removal and use of tissues as described below. Colour flow duplex imaging and ascending phlebography were used to identify venous reflux. All patients had an incompetent long saphenous vein up to the calf region, varicose tributaries and perforating veins. The long saphenous vein in the ankle region was competent in all cases. Except for one case of breast cancer and one of struma, the women were devoid of major diseases, in particular none suffered from diabetes, hypertension, or vasculitis. Four women had given birth to two children, and three women to one child; two of them were taking steroid contraceptives. Short pieces of tissue were carefully removed before starting the stripping procedure from three anatomical locations (Figure 1): proximal long saphenous vein in the inguinal area about 5 mm distal to its junction with the femoral vein (tissue A); distal long saphenous vein just above the medial ankle (tissue B); the third tissue was a rather large tributary to the long saphenous vein just below the knee (C). Tissue A was incompetent with little or no dilatation, tissue B was grossly nonvaricose, competent and appeared clinically normal, tissue C was always dilated, overtly varicose and incompetent. In a few cases, tissue A was incompetent and the vein was enlarged and varicose; these were not included in group A but formed a separate group that is referred to in the text.
Figure 1.
Procurement of variously diseased vein tissues from 3 anatomical locations (modified from [7]). Tissue A was a morphologically incompetent but nonvaricose segment of proximal long saphenous vein from the groin; tissue B, from distal long saphenous vein above the ankle, was clinically nonvaricose with competent valves; tissue C, from a tributary to the long saphenous vein in the calf, was always overtly varicose and tortuous.
Organ bath experiments
The vein segments (∼1–3 cm in length) were transported to the laboratory in chilled buffer, cleaned of extraneous fat and connective tissue and cut into small strips 2–3 mm wide with the circular muscle layer intact. Care was taken not to damage the endothelium. Because of the larger calibre of the vein at locations A and C than B, strips A and C were longer (∼15 mm) than strips B (∼10 mm). Strips were mounted in 5 ml organ baths filled with warmed (37 °C) Krebs-Henseleit buffer containing 2.5 mmol l−1 calcium and gassed with carbogen (mixture of 95% O2, 5% CO2). Isotonic changes in length were measured by a mechano-electronic transducer and continuously recorded on Watanabe Multicorders (Hugo Sachs-Elektronik, Freiburg, Germany). The strips stabilized in length within 2 h after incubation at about 50% of the maximum shortening (which was determined by addition of 80 mmol l−1 K+) and remained constant throughout the experiment.
Experimental protocol
Phenylephrine was added to equilibrated tissues in individual doses to give final bath concentrations of 0.1–100 µmol l−1, allowing 3–5 min for each concentration. After the last dose, acetylcholine (0.01–10 µmol l−1) was added (∼2 min for each concentration) and the relaxation recorded. The agonists were removed by repeated washes with Krebs-Henseleit buffer until the baseline length of strips was regained. Aescin (1–100 µg ml−1, equivalent to 0.9–90 µmol l−1) was then tested in a similar manner, requiring 20–30 min for each concentration (plasma concentrations of aescin in humans after administration of aesculus extract-containing preparations are ∼10–20 ng ml−1 [11]). Finally, 80 mmol l−1 KCl was added to obtain the reference maximum contraction (= control in Figures 2 and 3). The experiments with sodium nitroprusside were done separately. Tissues were contracted with the thromboxane mimetic U 46619 and angiotensin II (both 50 nmol l−1) until a similar degree of shortening of vessel strips was obtained as with phenylephrine, followed by addition of a maximally active concentration of sodium nitroprusside (30 µmol l−1). We have found repeatedly that the addition of angiotensin II to U 46619 gives a quicker and more stable contraction than the thromboxane mimetic alone.
Figure 2.
Contractile response to phenylephrine of vein strips derived from anatomical locations A (○), B (•) and C (□) (compare Figure 1). Results are expressed as percent of reference contraction obtained with 80 mmol l−1 KCl at the end of experiment (equivalent to 10.5 ± 1.5 g in tissues A and 7.6 ± 1.0 g in tissues B using isometric registration). Data points represent the mean ± s.e.mean of 30 tissues (A), 19 tissues (B) and 15 tissues (C) derived from seven patients in each case. The mean maximum contraction was not different between groups A and B, but was significantly smaller in group C (see Table 1).
Figure 3.
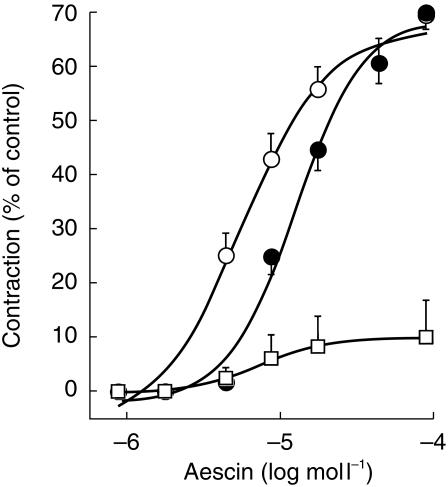
Contractile response to aescin of vein strips derived from anatomical locations A (○), B (•) and C (□). For details, see Figures 1 and 2. Data points represent the mean±s.e.mean of 28 tissues (A), 12 tissues (B) and 16 tissues (C) derived from six patients in each case (in one patient, tissues were derived from the right and left leg, with varicosity in both extremities).
Exclusion criteria
All strips from group A contracted to depolarization by excess KCl at the end of experiment and were included in the analysis. Two strips of tissue B out of a total of 21 obtained did not readily react to K+ depolarization, probably due to damage during excision, and were discarded. The decision was based on the observation that in both cases, at least one adjacent B strip showed normal contractility, excluding the possibility that the vessel was already functionally defective before excision. All strips of group C were included (most of them showed no contraction, see Results).
Data analysis
Data are presented as arithmetic means± s.e.mean. Concentration-effect curves were fitted individually yielding EC50 values (molar concentration of the agonist that produces 50% of the maximum effect, a measure of drug potency) and the corresponding maximum effect values (maximum effect [Emax]: percent of KCl-induced contraction, a measure of drug efficacy). EC50 values were calculated with the Apple Macintosh KaleidaGraph programme 3.0.8. The experimental curves were fitted nonlinearly to the Hill equation describing log-linear concentration-response curves [12]. Data were analysed by Student's t-test for unpaired observations, and mean differences (and 95% confidence limits) between the EC50 and Emax values for the different tissues were derived.
Materials
l-phenylephrine hydrochloride, acetylcholine chloride, sodium nitroprusside, angiotensin II and U 46619 (9,11-dideoxy-11α,9α-epoxymethanoprostaglandin F2α) were from were Sigma Chemicals (Vienna, Austria); aescin (C55H86O24Na; active ingredient of ReparilR) was from Madaus (Cologne, Germany). All other chemicals were of standard grade.
Results
Phenylephrine contracted veins from the inguinal (tissue A) and ankle area (tissue B) with similar potency and efficacy, but was virtually without effect in the overtly varicose segments from the calf (tissue C) (Figure 2). The corresponding EC50 and maximum effect values derived from nonlinear curve fits are listed in Table 1. Occasionally, the long saphenous vein of the groin area showed signs of valvular incompetence and the vein wall was thinned. The reactivity of these tissues toward phenylephrine was similar to that of tissues A (EC50: 1.1 µmol l−1, Emax: 71% of control; n = 8 strips from two patients; not shown). Likewise, a greatly varicose tributary to the long saphenous vein in the groin area was similarly reactive (EC50: 1.3 µmol l−1; Emax: 83 ± 10% of control; n = 4 strips from one patient; not shown). The contractile effect of aescin is shown in Figure 3. The glycoside contracted strips A and B to a similar extent, but its potency was significantly higher in varicose than nonvaricose vein (factor: 1.7; P < 0.05). The reactivity of segments of overtly varicose veins was again close to zero (Table 1).
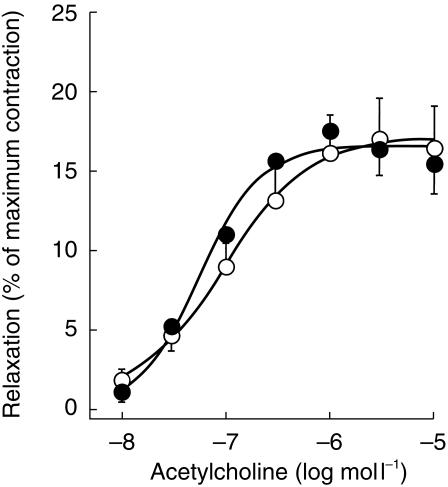
Table 1.
Contractile and relaxant responses of lower limb vein tissues from patients with primary varicosity.
| Emax (%) | Difference (95% CI) | EC50 (µmol l−1) | Difference (95% CI) | |
|---|---|---|---|---|
| Phenylephrine | ||||
| A (n = 30) | 76 ± 3 | 2.9 ± 0.3 | ||
| B (n = 19) | 70 ± 4 | 7 (6, 8)# | 2.5 ± 0.5 | 0.4 (0, 0.8) |
| C (n = 15) | 5 ± 5⋆ | 65 (63, 67)# | – | |
| Aescin | ||||
| A (n = 28) | 70 ± 2 | 9.4 ± 1.0† | ||
| B (n = 12) | 71 ± 3 | −1 (−2, 0) | 15.9 ± 2.5 | −6.5 (−7,3, −5.7)# |
| C (n = 16) | 10 ± 7⋆ | 61 (59, 63)# | – | |
| Acetylcholine | ||||
| A (n = 11) | 18 ± 3 | 0.1 ± 0.05 | ||
| B (n = 8) | 17 ± 2 | 1 (−1, 3) | 0.1 ± 0.02 | 0.1 (−0.1, 0.3) |
| C (n = 17) | 2 ± 1⋆ | 15 (14, 16)# | – | |
A, B, and C refer to the three anatomical locations shown in Figure 1. Values are means ± s.e.mean or (95% confidence limits, CI). n refers to the number of tissues studied; the number of patients from which they were derived is given in the figure legends. Maximum response (Emax,%) was defined with excess KCl. Difference refers to mean differences in Emax and EC50 values with 95% confidence limits between records obtained in tissues A vs B (EC50 and Emax) and B vs C (Emax).
P < 0.05 vs A or B
P < 0.05 vs B
the 95% confidence limit excludes zero.
Figure 4.
Relaxant response to acetylcholine in long saphenous vein strips derived from anatomical locations A (○)and B (•). For details, see Figures 1 and 2. Results are percent of initial maximum contraction obtained with phenylephrine (10 µmol l−1). Data points represent the mean±s.e.mean of 11 tissues (A) and 8 tissues (B) derived from six patients.
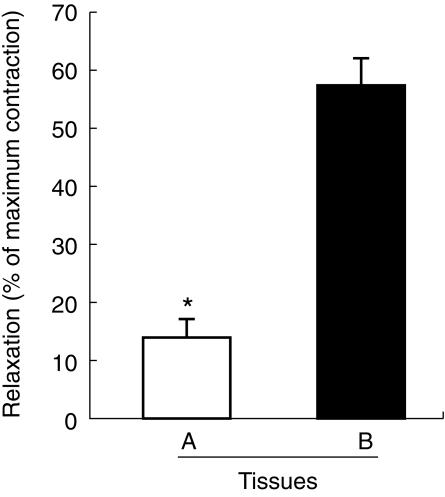
The contractile agents phenylephrine (used to test acetylcholine relaxation) and U 46619 plus angiotensin II (used to test sodium nitroprusside relaxation) were effective in all tissues from locations A and B, but generally not in tissue C, as expected from the results with phenylephrine and aescin (see above). Following precontraction, acetylcholine relaxed vein segments from the locations A and B with similar potency and efficacy, and the respective EC50 and Emax values are also listed in Table 1. When added to saphenous vein segments from the groin that were more heavily diseased (dilated and varicose; see section on patients and test groups), acetylcholine was similarly potent and efficacious as in tissues A (EC50: 0.09 ± 0.01 µmol l−1; Emax 18 ± 2% of control; n = 3; not shown). The relaxation response to sodium nitroprusside (30 µmol l−1) was greater in the nonvaricose tissues B (58 ± 4% of control) than in the incompetent tissues A (14 ± 3%; Figure 5). A few overtly varicose segments (C) contracted to the combination of U 46619 and angiotensin II (6 out of 24 strips), but the maximum contraction was much less than in tissues B and C, preventing the quantification of sodium nitroprusside or acetylcholine effects.
Figure 5.
Relaxant response to sodium nitroprusside (30 µmol l−1) in vein strips derived from anatomical locations A and B. Results are percent of initial maximum contraction obtained with U 46619 and angiotensin II (each 50 nmol l−1). Data points represent the mean±s.e.mean of 17 tissues (A) and 8 tissues (B) derived from six patients.
Discussion
In overtly varicose tributaries of human long saphenous vein vasomotion in response to contractile and relaxant stimuli is largely lost. Vein segments from the groin and ankle region showed similar reactivity, although the inguinal vessel was incompetent and the segment from the ankle was clinically healthy. Therefore, it appears that pharmacological treatment regimens aimed at raising venous tone can be successful in early, but not late stages of the disease.
There is general agreement that varicosity needs to be treated to prevent leg ulcers, and both surgical procedures and pharmacotherapy have long been used clinically. Currently, the pharmacotherapeutic approaches are aimed at reducing microvascular disturbances including excessive capillary filtration leading to leg oedema, and/or increasing venous tone which would reduce venous thrombosis [10]. However, an important question is: how effective are venotonic drugs in raising venous tone, and to what extent does this contribute to the clinical effectiveness of the treatment? To test the contractile response of the saphenous vein tissues we used the two agonists, phenylephrine and aescin. Phenylephrine activates postjunctional α-adrenergic receptors, and aescin is a phlebotonic drug often used to improve symptoms of chronic venous insufficiency. The mechanism of action of which is not well understood [13]. The glycoside has anti-inflammatory activity, prevents oedema formation by decreasing the permeability of the vessel wall for different low and high molecular weight plasma components [14] and exerts venoconstriction [9]. Both phenylephrine and aescin contracted tissues from the ankle and groin regions (A and B, respectively), but were virtually ineffective in the saphenous vein tributaries with overt tortuosities (segments C). These findings show that in vein segments with the severest disease, smooth muscle contractility is lost and ‘venotonic’ drugs cannot be expected to counteract the overt chronic dilatation typically seen on the calf and thigh of patients with primary varicosity. Hence, venous return from the lower extremities becomes largely independent of vein size in such patients and probably depends mostly on skeletal muscle contractions. On the other hand, venoconstriction to the two agonists was intact in segments from the groin, indicating that phlebotonic drugs such as aescin may raise venous tone in segments that are morphologically still relatively little affected by the disease, i.e. if the cellular contraction mechanism is still intact. This supports early intervention to prevent the progression of the disease.
Although we and others have previously reported reduced contraction in varicose veins [6–7,15–16], in all studies saphenous veins from patients who underwent arterial bypass grafting procedures were used as ′controls'. This contrasts with the present study, where we have used clinically healthy and varicose vein segments from the same patient.
Relaxation of the venous wall, even when caused by valvular insufficiency, aggravates venous stasis and oedema. We have previously shown [8] that cultured endothelial cells from varicose veins derived from the inguinal area had a higher basal production of cGMP than cells derived from clinically healthy veins prepared for bypass surgery. Similarly, nitroprusside, an endothelium-independent relaxant in human saphenous vein, was more potent in relaxing diseased saphenous veins and the response to the agonist was greater in this tissue than in the clinically healthy control tissues from the bypass patients. Individually or in combination these findings suggested stronger vascular relaxation in primary varicosity [8]. In the present study, we compared the relaxant response to sodium nitroprusside and acetylcholine, an endothelium-dependent agonist in human saphenous vein [17–18], in variously diseased tissues from the same patient. Acetylcholine relaxed tissues A and B similarly, indicating that this agonist is unlikely to aggravate the early phase of varicosity, mainly characterized by the incompetence of the vessel (tissue A). In the final stage of the disease (tissue C), both contraction and relaxation are lost.
In conclusion, the present findings further support the hypothesis that abnormalities within the venous wall affect venous smooth muscle contractility. Because both competent and incompetent clinically normal vessels show normal contractile responses, whereas varicose vessels no longer respond to vascular control, pharmacological treatment regimens may be successful in early, but not in the late stages of the disease. Whether the observed loss of vasoconstrictor effects is a cause or a sequel to the changes in the varicose vein wall requires additional investigation.
Acknowledgments
The authors acknowledge the technical assistance of Mrs Birgit Jelinek-Fink. This work was supported by the Austrian Research Fund, projects 12934 and 13013 (to F. B.).
References
- 1.Callam MJ. Epidemiology of varicose veins. Br J Surg. 1994;81:167–173. doi: 10.1002/bjs.1800810204. [DOI] [PubMed] [Google Scholar]
- 2.Vanhoutte PM, Corcaud S, de Montrion C. Venous disease: from pathophysiology to quality of life. Angiology. 1997;48:559–567. doi: 10.1177/000331979704800702. [DOI] [PubMed] [Google Scholar]
- 3.Dalen JE, Alpert JS. Natural history of pulmonary embolism. Progr Cardiovasc Dis. 1975;17:257–270. doi: 10.1016/s0033-0620(75)80017-x. [DOI] [PubMed] [Google Scholar]
- 4.Callam MJ. Prevalence of chronic venous leg ulceration and severe chronic venous disease in Western Countries. Phlebology. 1992;7(Suppl 1):6–12. [Google Scholar]
- 5.Thulesius O, Said S, Shuhaiber H, Neglen P, Gjores JE. Endothelial mediated enhancement of noradrenaline induced vasoconstriction in normal and varicose veins. Clin Physiol. 1991;11:153–159. doi: 10.1111/j.1475-097x.1991.tb00108.x. [DOI] [PubMed] [Google Scholar]
- 6.Blöchl-Daum B, Schuller-Petrovic S, Wolzt M, Korn A, Böhler K, Eichler H-G. Primary defect in α-adrenergic responsiveness in patients with varicose veins. Clin Pharmacol Ther. 1991;49:49–52. doi: 10.1038/clpt.1991.9. [DOI] [PubMed] [Google Scholar]
- 7.Lowell R, Gloviczki P, Miller V. In vitro evaluation of endothelial and smooth muscle function of primary varicose veins. J Vasc Surg. 1992;16:679–686. [PubMed] [Google Scholar]
- 8.Schuller-Petrovic S, Siedler S, Kern T, Meinhart J, Schmidt K, Brunner F. Imbalance between the endothelial cell-derived contracting factors prostacyclin and angiotensin II and nitric oxide/cyclic GMP in human primary varicosis. Br J Pharmacol. 1997;122:772–778. doi: 10.1038/sj.bjp.0701437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Annoni F, Mauri A, Marincola F, Resele LF. Venotonic activity of escin on the human saphenous vein. Arzneim-Forsch (Drug Res) 1979;29:672–675. [PubMed] [Google Scholar]
- 10.Jain KK. Pathophysiology and pharmacotherapy of chronic venous insufficiency: a critical review. J Clin Res. 1998;1:269–288. [Google Scholar]
- 11.Kunz K, Lorkowski G, Petersen G, Samcova E, Schaffler K, Wauschkuhn CH. Bioavailability of escin after administration of two oral formulations containing aescullus extract. Arzneim-Forsch (Drug Res) 1998;48:822–825. [PubMed] [Google Scholar]
- 12.Barlow R, Blake JF. Hill coefficients and the logistic equation. Trends Pharmacol Sci. 1989;10:440–441. doi: 10.1016/S0165-6147(89)80006-9. [DOI] [PubMed] [Google Scholar]
- 13.Pittler MH, Ernst E. Horse-chestnut seed extract for chronic venous insufficiency. Arch Dermatol. 1998;134:1356–1360. doi: 10.1001/archderm.134.11.1356. [DOI] [PubMed] [Google Scholar]
- 14.Rehn D, Unkauf M, Klein P, Jost V, Lücker PW. Comparative clinical efficacy and tolerability of oxerutins and horse chestnut extract in patients with chronic venous insufficiency. Arzneim-Forsch (Drug Res) 1996;46:483–487. [PubMed] [Google Scholar]
- 15.Barber DA, Wang X, Gloviczki P, Miller VM. Characterization of endothelin receptors in human varicose veins. J Vasc Surg. 1997;26:61–69. doi: 10.1016/s0741-5214(97)70148-4. [DOI] [PubMed] [Google Scholar]
- 16.Rizzi A, Quaglio D, Vasquez G, et al. Effects of vasoactive agents in healthy and diseased human saphenous veins. J Vasc Surg. 1998;28:855–861. doi: 10.1016/s0741-5214(98)70061-8. [DOI] [PubMed] [Google Scholar]
- 17.Schwartz LB, Radic ZS, O'Donohoe MK, Mikat EM, McCann RL, Hagen P-O. Saphenous vein endothelium-dependent relaxation in patients with peripheral vascular disease. Ann Vasc Surg. 1992;6:425–432. doi: 10.1007/BF02006997. [DOI] [PubMed] [Google Scholar]
- 18.Chua YL, Pearson PJ, Evora PRB, Schaff HV. Detection of intraluminal release of endothelium-derived relaxing factor from human saphenous veins. Circulation. 1993;88:II-128–II-132. [PubMed] [Google Scholar]