Abstract
Aims
To determine the cytochrome P450 (CYP) isoforms involved in the oxidation of propofol by human liver microsomes.
Methods
The rate constant calculated from the disappearance of propofol in an incubation mixture with human liver microsomes and recombinant human CYP isoforms was used as a measure of the rate of metabolism of propofol. The correlation of these rate constants with rates of metabolism of CYP isoform-selective substrates by liver microsomes, the effect of CYP isoform-selective chemical inhibitors and monoclonal antibodies on propofol metabolism by liver microsomes, and its metabolism by recombinant human CYP isoforms were examined.
Results
The mean rate constant of propofol metabolism by liver microsomes obtained from six individuals was 4.2 (95% confidence intervals 2.7, 5.7) nmol min−1 mg−1 protein. The rate constants of propofol by microsomes were significantly correlated with S-mephenytoin N-demethylation, a marker of CYP2B6 (r = 0.93, P < 0.0001), but not with the metabolic activities of other CYP isoform-selective substrates. Of the chemical inhibitors of CYP isoforms tested, orphenadrine, a CYP2B6 inhibitor, reduced the rate constant of propofol by liver microsomes by 38% (P < 0.05), while other CYP isoform-selective inhibitors had no effects. Of the recombinant CYP isoforms screened, CYP2B6 produced the highest rate constant for propofol metabolism (197 nmol min−1 nmol P450−1). An antibody against CYP2B6 inhibited the disappearance of propofol in liver microsomes by 74%. Antibodies raised against other CYP isoforms had no effect on the metabolism of propofol.
Conclusions
CYP2B6 is predominantly involved in the oxidation of propofol by human liver microsomes.
Keywords: cytochrome P4502B6, liver, metabolism, propofol
Introduction
Propofol is a short-acting anaesthetic commonly used in clinical practice. It is rapidly eliminated from the body, and glucuronidation in the liver is the major pathway of metabolism [1–3]. However, the oxidation of propofol via ring hydroxylation [3] accounts for approximately 40% of the dose [1], and this reaction is catalysed by cytochrome P450 (CYP) [4]. Propofol is frequently used in combination with other agents such as opioids and other anaesthetics, and has been shown to inhibit their metabolism and those of those compounds [5–9]. Since inhibition of the metabolism of simultaneously administered drugs is often observed when both agents are metabolized by the same CYP isoforms [10, 11], it is important to elucidate the CYP isoforms involved in the metabolism of propofol to predict possible CYP-mediated drug interactions between this with other agents. Although there have been a large number of reports regarding the pharmacokinetics of propofol [1–3], few studies have been performed to elucidate the hepatic CYP isoforms involved in its metabolism [4]. The aim of the present study was to elucidate the CYP isoforms involved in the metabolism of propofol using human liver microsomes, recombinant human CYP isoforms and selective CYP antibodies in vitro.
Methods
Materials
The study protocol was approved by the Institutional Human Investigational Committee, Osaka City University Medical School, Osaka, Japan. Propofol was obtained from Aldrich (Milwaukee, WI, USA). Reduced nicotinamide adenine dinucleotide phosphate (NADPH), phenacetin, paracetamol (acetaminophen), thymol, orphenadrine, quinidine, diethyldithiocarbamate (DDC) and troleandomycin (TAO) were obtained from Sigma Chemical Co. (St Louis, MO, USA). Midazolam and 1′-hydroxymidazolam (1′-OH MDZ) were kind gifts from Hoffmann-La Roche Ltd. (Nutley, NJ, USA). S-mephenytoin, nirvanol, 4′-hydroxymephenytoin and furafylline were obtained from Ultrafine Chemicals (Manchester, UK). Sulfaphenazole was a kind gift from Meiji Yakuhin Co. Ltd (Tokyo, Japan). Human liver microsomes were obtained from the International Institute for the Advancement of Medicine (Scranton, PA, USA). Recombinant human P450s expressed in human lymphoblast cells with NADPH-cytochrome P450 reductase were obtained from Gentest (Woburn, MA, USA). Monoclonal antibodies raised against CYP1A2, 2B6, 3A4 and a polyclonal antibody raised against CYP2C were also obtained from Gentest. The selectivities and inhibitory activities of these antibodies were confirmed and described in the accompanying instruction manuals. Antibody raised against CYP2C inhibits both CYP2C9 and CYP2C19 activities. A reverse-phase octadecasilyl column used for high-performance liquid chromatography (h.p.l.c.) was obtained from the Tosoh Corp. (Tokyo, Japan). Other reagents and organic solvents were obtained from Wako Pure Chemical Industries (Osaka, Japan).
Incubation conditions
The incubation mixture consisted of human liver microsomes containing 0.1 mg protein, 4 mm NADPH, and propofol in the presence or absence of one of the chemical inhibitors of CYP isoforms in 100 mm potassium phosphate buffer (pH 7.4) made up to a final volume of 0.5 ml. When recombinant CYP isoforms were used instead of liver microsomes, the incubations contained 30 pmol CYP. Propofol was initially prepared in methanol solution, and the final concentration in the incubation mixture was adjusted to 20 µm. The final concentration of methanol was less than 0.4%. The concentrations of propofol used in our study were based on those observed in clinical practice [7].
The mixture was incubated at 37 °CC for 0, 5, 10 and 15 min. Incubations were performed in individual tubes for each time point. When recombinant CYP2B6 was used, 2 pmol CYP was incubated with propofol and NADPH for 0, 2, 4 and 6 min. Incubations were terminated by addition of 1 m NaOH (50 µl) and cooled on ice. Thymol (10 µl of a 6 mm in methanol solution) as an internal standard and ethyl acetate 1 ml were added to the mixture, which was then vortexed for 10 min and centrifuged at 3000 rev min−1 for 10 min. An aliquot of the ethyl acetate layer (80 µl) was mixed with 160 µl of the h.p.l.c. mobile phase, and 150 µl of this mixture was injected onto the h.p.l.c. system described by Plummer [12]. The limit of detection of propofol was 0.05 µm. The intra- and inter- assay coefficients variation measured were 4.5 and 6.2% at 0.1 µm and 3.5 and 4.0% at 10 µm, respectively (n = 6 replicates).
Measurement of the disappearance rate constant for propofol metabolism and of the metabolic activity of CYP-selective substrates
The rate constant of propofol metabolism by human microsomes and recombinant CYP isoforms was estimated from its initial concentration in the incubation medium (C) and the half-time of substrate disappearance (t½) using the following equation [13]:
The half-life of substrate disappearance was calculated from linear regression analysis of semilogarithmic plots of the mean of the residual concentrations of propofol of three experiments at each incubation time. Phenacetin O-deethylation, S-mephenytoin N-demethylation and 4′-hydroxylation and midazolam 1′-hydroxylation activities in human liver microsomes obtained from 11 individuals were quantified to measure CYP1A2, 2B6, 2C19 and 3A4 activities, respectively, using previously reported methods [11, 14–16].
Studies with chemical inhibitors and inhibitory antibodies
The effects of a series of CYP isoform-selective inhibitors on the metabolism of propofol were examined using liver microsomes obtained from six individuals. Chemical inhibitors were used at the following final concentrations: furafylline (CYP1A2 inhibitor, 20 µm), orphenadrine (CYP2B6 inhibitor, 50 µm), sulfaphenazole (CYP2C9 inhibitor, 20 µm), quinidine (CYP2D6 inhibitor, 5 µm), DDC (CYP2E1 inhibitor, 100 µm) and TAO (CYP3A inhibitor, 100 µm). The concentrations of the chemical inhibitors employed in the present study were similar to those reported previously [17, 18]. Furafylline, orphenadrine and troleandomycin were preincubated for 15 min with microsomes and NADPH prior to addition of substrate. Experiments with inhibitory monoclonal antibodies were performed as reported previously [19].
Statistical methods
Statistical comparisons were made using unpaired t-test. the relationships between the rate constant for propofol metabolism and phenacetin O-deethylation, S-mephenytoin N-demethylation and 4′-hydroxylation and midazolam 1′-hydroxylation activities were determined by linear regression analysis using StatView ver 4.5 (Abacus Concepts Inc., CA, USA). All values are presented as means (95% confidence intervals). P values < 0.05 were considered statistically significant.
Results
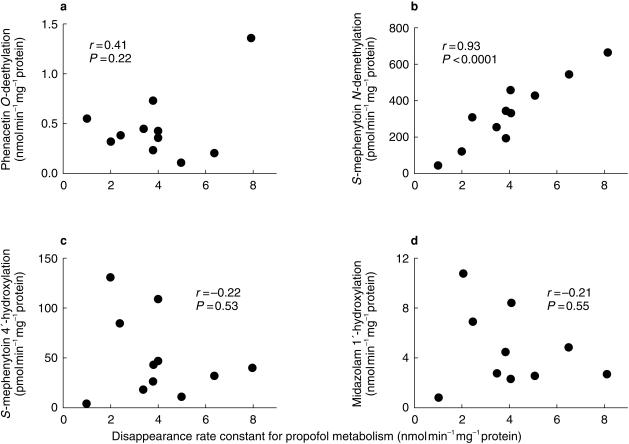
There was an inverse linear correlation between incubation time and the logarithm of the concentration of propofol remaining in the mixture when initial substrate concentrations were between 0.5 and 50 µm, incubation times were 15 min or less and the concentrations of microsomal protein and CYP were less than 0.2 mg and 50 pmol, respectively, which is indicative of first-order kinetics. The mean rate constant for propofol metabolism by human liver microsomes obtained from six individuals was 4.2 (2.7, 5.7) nmol min−1 mg−1 protein. The rate constants correlated significantly with S-mephenytoin N-demethylation activity, a marker of CYP2B6 (r = 0.93, P < 0.0001), but not with phenacetin O-deethylation activity, a marker of CYP1A2 (r = 0.41, P = 0.22), nor with S-mephenytoin 4′-hydroxylation activity, a marker of CYP2C19 (r = −0.22, P = 0.53), or midazolam 1′-hydroxylation activity, a marker of CYP3A4 (r = −0.21, P = 0.55) (Figure 1). The mean rate constant for propofol metabolism by liver microsomes from six individuals incubated with orphenadrine was 2.6 (1.8, 3.4) nmol min−1 mg−1 protein, which was significantly lower than the control value without inhibitors (P < 0.05). Mean data from incubations containing furafylline, sulfaphenazole, quinidine, DDC and TAO were 3.6 (1.9, 5.3), 4.4 (2.7, 6.1), 5.2 (3.9, 6.5), 4.0 (2.7, 5.3) and 4.6 (3.2, 6.0), respectively. None of these was significantly different from the control value.
Figure 1.
Correlations of the disappearance rate constant for propofol metabolism with (a) phenacetin O-deethylation, a marker of CYP1A2, (b) S-mephenytoin N-demethylation, a marker of CYP2B6, (c) S-mephenytoin 4′-hydroxylation, a marker of CYP2C19 and (d) midazolam 1′-hydroxylation, a marker of CYP3A4, by human liver microsomes from 11 individuals. Concentrations of phenacetin, S-mephenytoin and midazolam were 200 µm, 1 mm and 10 µm, respectively. The amount of microsomal protein was 400 µg and incubation time was 20 min. Each plot depicts the mean of three experiments.
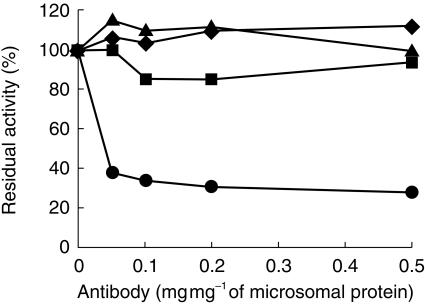
The rate constants for propofol metabolism by recombinant CYP1A2, 2B6, 2C9 and 2C19 were 11.2, 26.6, 0.2 and 2.8 nmol min−1 mg−1 protein (54.8, 196.8, 0.54 and 7.8 nmol min−1 nmol−1 P450) (mean of two experiments). CYP2A6, 2D6, 2E1 3A4 and 3A5 showed no detectable activity towards propofol. Experiments using inhibitory antibodies raised against CYP1A2, 2B6, 2C and 3A4 were also used to determine the contribution of these CYP isoforms to the metabolism of propofol by human liver microsomes. Of these only the CYP2B6 antibody inhibited its metabolism, decreasing the rate constant by 74% (mean of two experiments). Antibodies raised against CYP1A2, 2C and 3A4 had no effect (Figure 2).
Figure 2.
Effect of antibodies raised against CYP1A2 (▪), 2B6 (•), 2C (▴) and 3A4 (♦) on the disappearance rate constant for propofol metabolism by human liver microsomes. The concentration of propofol was 20 µm. The amount of protein in liver microsomes in each incubation mixture was 100 µg. Microsomes and antibodies were preincubated at room temperature for 20 min, followed by addition of propofol and NADPH. The total amount of protein in the antibodies and preimmune immunoglobulin G was 50 µg. The results of duplicate determinations at each concentration of antibodies added to microsomes are shown. The mean (95% CI) rate constant for propofol metabolism in the absence of antibodies was 4.2 (2.7, 5.7) nmol min−1 mg−1 protein.
Discussion
The activity of CYP isoforms has traditionally been examined by measuring the rates of formation of metabolites formed from the drug of interest. In our experiments, we studied the CYP isoforms involved in the metabolism of propofol by monitoring the rate of disappearance of the parent drug from an incubation mixture containing human liver and recombinant human CYP microsomes [13]. This method is useful for assessing the overall elimination of a drug, particularly when its metabolic profile is not completely known [20], or when a metabolite is unstable or difficult to measure.
Several lines of evidence obtained in the present study suggest that CYP2B6 is the principal CYP isoform involved in the metabolism of propofol by human liver: (1) the disappearance rate constants of propofol by 11 different human liver microsomes were highly correlated with S-mephenytoin N-demethylation activity, a marker of CYP2B6, (2) orphenadrine, an inhibitor of CYP2B6, significantly inhibited the metabolism of propofol, (3) recombinant human CYP2B6 exhibited the highest propofol metabolic activity towards propofol compared with the other recombinant CYPs tested and (4) propofol metabolism by human liver microsomes was inhibited by a monoclonal antibody raised against CYP2B6.
Recombinant CYP1A2, 2C9 and 2C19 also metabolized propofol, but at a lower rate than CYP2B6. Furthermore, chemical inhibitors of CYP1A2 and 2C9 activity or antibodies raised against these CYP isoforms had no effect on propofol metabolism. Guitton et al. [4] have suggested that CYP2C9 plays a role in the metabolism of propofol. However, CYP2B6 was not examined in their study, probably because the content of CYP2B6 in human liver microsomes is low [21]. The contribution of CYP2B to the hydroxylation of propofol has also been established in experimental animals [22].
The elimination of propofol from the body after intravenous injection depends primarily on hepatic blood flow rather than on metabolic activity in the liver, since its hepatic extraction ratio is more than 70% [23]. However, identifying the specific enzymes involved in the metabolism of propofol is required to elucidate potential metabolic interactions between propofol and other agents. We have shown recently that propofol competitively inhibits the metabolism of midazolam [7], which is selectively metabolized by CYP3A4 both in vitro and in vivo [24]. Competitive inhibition of metabolism is often observed when two agents are metabolized by the same CYP isoforms [10, 11]. However, the present in vitro study revealed that CYP3A4 was not involved in the metabolism of propofol. The reasons for the discrepancies in findings between our recent and the present study remain unknown. However, it is possible that CYP2B6 is involved to a certain extent in the metabolism of midazolam, since CYP2B6 has significant metabolic activity towards some substrates thought to be catalysed predominantly by CYP3A4, such as lignocaine and dextromethorphan as well as midazolam [25–27].
In summary, we have shown that CYP2B6 is the predominant CYP isoform involved in the oxidation of propofol by human liver microsomes.
Acknowledgments
This study was supported by the Fund for Medical Research from Osaka City University Medical Research Foundation (Osaka, Japan) and Grant-in-Aid for Research from the Ministry of Education, Science and Culture of Japan (No. 09771183 and no. 11671517, Tokyo, Japan).
References
- 1.Simons PJ, Cockshott ID, Douglas EJ, Gordon EA, Hopkins K, Rowland M. Disposition in male volunteers of a subanaesthetic intravenous dose of an oil in water emulsion of 14C-propofol. Xenobiotica. 1998;18:281–285. doi: 10.3109/00498258809041679. [DOI] [PubMed] [Google Scholar]
- 2.Gray PA, Park GR, Cockshott ID, Douglas EJ, Shuker B, Simons PJ. Propofol metabolism in man during the anhepatic and reperfusion phases of liver transplantation. Xenobiotica. 1992;22:105–114. doi: 10.3109/00498259209053107. [DOI] [PubMed] [Google Scholar]
- 3.Vree TB, Lagerwerf AJ, Bleeker CP, de Grood PMRM. Direct high-performance liquid chromatography determination of propofol and its metabolite quinol with their glucuronide conjugates and preliminary pharmacokinetics in plasma and urine of man. J Chromatogr B. 1999;721:217–228. doi: 10.1016/s0378-4347(98)00466-6. [DOI] [PubMed] [Google Scholar]
- 4.Guitton J, Buronfosse T, Desage M, et al. Possible involvement of multiple human cytochrome P450 isoforms in the liver metabolism of propofol. Br J Anaesth. 1998;80:788–795. doi: 10.1093/bja/80.6.788. [DOI] [PubMed] [Google Scholar]
- 5.Janicki PK, James MFM, Erskine WAR. Propofol inhibits enzymatic degradation of alfentanil and sufentanil by isolated liver microsomes in vitro. Br J Anaesth. 1992;68:311–312. doi: 10.1093/bja/68.3.311. [DOI] [PubMed] [Google Scholar]
- 6.Pavlin DJ, Coda B, Shen DD, et al. Effects of combining propofol and alfentanil on ventilation, analgesia, sedation, and emesis in human volunteers. Anesthesiology. 1996;84:23–37. doi: 10.1097/00000542-199601000-00004. [DOI] [PubMed] [Google Scholar]
- 7.Hamaoka N, Oda Y, Hase I, et al. Propofol decreases the clearance of midazolam by inhibiting CYP3A4: An in vivo and in vitro study. Clin Pharmacol Ther. 1999;66:110–117. doi: 10.1053/cp.1999.v66.100038001. [DOI] [PubMed] [Google Scholar]
- 8.Chen TL, Ueng TH, Chen SH, Lee PH, Fan SZ, Liu CC. Human cytochrome P450 mono-oxygenase system is suppressed by propofol. Br J Anaesth. 1995;74:558–562. doi: 10.1093/bja/74.5.558. [DOI] [PubMed] [Google Scholar]
- 9.Chen TL, Wu CH, Chen TG, Tai YT, Chang HC, Lin CJ. Effects of propofol on functional activities of hepatic and extrahepatic conjugation enzyme systems. Br J Anaesth. 2000;84:771–776. doi: 10.1093/oxfordjournals.bja.a013592. [DOI] [PubMed] [Google Scholar]
- 10.Serlin MJ, Sibeon RG, Mossman S, et al. Cimetidine: interaction with oral anticoagulants in man. Lancet. 1979;2:317–319. doi: 10.1016/s0140-6736(79)90340-4. [DOI] [PubMed] [Google Scholar]
- 11.Oda Y, Mizutani K, Hase I, Nakamoto T, Hamaoka N, Asada A. Fentanyl inhibits metabolism of midazolam: competitive inhibition of CYP3A4 in vitro. Br J Anaesth. 1999;82:900–903. doi: 10.1093/bja/82.6.900. [DOI] [PubMed] [Google Scholar]
- 12.Plummer GF. Improved method for the determination of propofol in blood by high-performance liquid chromatography with fluorescence detection. J Chromatogr. 1987;421:171–176. doi: 10.1016/0378-4347(87)80394-8. [DOI] [PubMed] [Google Scholar]
- 13.Svensson USH, Ashton M. Identification of the human cytochrome P450 enzymes involved in the in vitro metabolism of artemisinin. Br J Clin Pharmacol. 1999;48:528–535. doi: 10.1046/j.1365-2125.1999.00044.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tassaneeyakul W, Birkett DJ, Veronese ME, et al. Specificity of substrate and inhibitor probes for human cytochromes P450 1A1 and 1A2. J Pharmacol Exp Ther. 1993;265:401–407. [PubMed] [Google Scholar]
- 15.Heyn H, White RB, Stevens JC. Catalytic role of cytochrome P4502B6 in the N-demethylation of S-mephenytoin. Drug Metab Dispos. 1996;24:948–954. [PubMed] [Google Scholar]
- 16.Wrighton SA, Stevens JC, Becker GW, VandenBranden M. Isolation and characterization of human liver cytochrome P450 2C19: Correlation between 2C19 and S-mephenytoin 4′-hydroxylation. Arch Biochem Biophys. 1993;306:240–245. doi: 10.1006/abbi.1993.1506. [DOI] [PubMed] [Google Scholar]
- 17.Reidy GF, Mehta I, Murray M. Inhibition of oxidative drug metabolism by orphenadrine: in vitro and in vivo evidence for isozyme-specific complexation of cytochrome P-450 and inhibition kinetics. Mol Pharmacol. 1989;35:736–743. [PubMed] [Google Scholar]
- 18.Thummel KE, Kharasch ED, Podoll T, Kunze K. Human liver microsomal enflurane defluorination catalyzed by cytochrome P-450 2E1. Drug Metab Dispos. 1993;21:350–357. [PubMed] [Google Scholar]
- 19.Oda Y, Furuichi K, Tanaka K, et al. Metabolism of a new local anesthetic, ropivacaine, by human hepatic cytochrome P450. Anesthesiology. 1995;82:214–220. doi: 10.1097/00000542-199501000-00026. [DOI] [PubMed] [Google Scholar]
- 20.Suzuki A, Iida I, Tanaka F, et al. Identification of human cytochrome P-450 isoforms involved in metabolism of R(+)- and S(−) -gallopamil: Utility of in vitro disappearance rate. Drug Metab Dispos. 1999;27:1254–1259. [PubMed] [Google Scholar]
- 21.Shimada T, Yamazaki H, Mimura M, Inui Y, Guengerich FP. Interindividual variations in human liver cytochrome P-450 enzymes involved in the oxidation of drugs, carcinogens and toxic chemicals: studies with liver microsomes of 30 Japanese and 30 Caucasians. J Pharmacol Exp Ther. 1994;270:414–423. [PubMed] [Google Scholar]
- 22.Hay Kraus BL, Greenblatt DJ, Venkatakrishnan K, Court MH. Evidence for propofol hydroxylation by cytochrome P4502B11 in canine liver microsomes: breed and gender differences. Xenobiotica. 2000;30:575–588. doi: 10.1080/004982500406417. [DOI] [PubMed] [Google Scholar]
- 23.Lange H, Stephan H, Rieke H, Kellermann M, Sonntag H, Bircher J. Hepatic and extrahepatic disposition of propofol in patients undergoing coronary bypass surgery. Br J Anaesth. 1990;64:563–570. doi: 10.1093/bja/64.5.563. [DOI] [PubMed] [Google Scholar]
- 24.Kronbach T, Mathys D, Umeno M, Gonzalez FJ, Meyer UA. Oxidation of midazolam and triazolam by human liver cytochrome P450IIIA4. Mol Pharmacol. 1989;36:89–96. [PubMed] [Google Scholar]
- 25.Ekins S, VandenBranden M, Ring BJ, et al. Further characterization of the expression in liver and catalytic activity of CYP2B6. J Pharmacol Exp Ther. 1998;286:1253–1259. [PubMed] [Google Scholar]
- 26.Imaoka S, Yamada T, Hiroi T, et al. Multiple forms of human P450 expressed in Saccharomyces cerevisiae. Systematic characterization and comparison with those of the rat. Biochem Pharmacol. 1996;51:1041–1050. doi: 10.1016/0006-2952(96)00052-4. [DOI] [PubMed] [Google Scholar]
- 27.Wang Y, Unadkat JD. Enzymes in addition to CYP3A4 and 3A5 mediate N-demethylation of dextromethorphan in human liver microsomes. Biopharm Drug Dispos. 1999;20:341–346. doi: 10.1002/(sici)1099-081x(199910)20:7<341::aid-bdd195>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]