Abstract
Aims
Methadone is predominantly metabolized by cytochrome P450 3A4 and the non nucleoside reverse transcriptase inhibitor (NNRTI) efavirenz is a recognized inducer of this enzyme. We evaluated the pharmacokinetics of methadone in the presence and absence of efavirenz when administered to HIV infected patients with a history of injection drug use (IDU).
Methods
Eleven patients on stable methadone maintenance therapy, due to commence antiretroviral therapy (ART), participated in this study. Steady state methadone kinetic profiles were obtained on two occasions 0, 1, 2, 3, 4, 5, 6, 7, 8 and 24 h post dosing. Following centrifugation, separated plasma was heated at 58 °C for 30 min to inactivate HIV and stored at −80 °C until methadone analysis by high performance liquid chromatography.
Results
When combined with efavirenz there was a marked decrease in the maximum plasma concentration (Cmax) of methadone from 689 (range 212–1568) to 358 (range 205–706) ng ml−1, P = 0.007:95% confidence interval (CI) 112–549. The mean area under the methadone concentration curve 0–24 h (AUC(0,24 h)) was also significantly reduced from 12341 (range 3682–34147) to 5309 (range 2430–10349) ng ml−1 h in the presence of efavirenz, P = 0.012:95% CI 1921–12143. Nine patients described symptoms of methadone withdrawal and received a dose increase. Although methadone AUC(0,24 h) was reduced by over 50% following efavirenz the mean increase in methadone dose required was 22% (range 15–30 mg).
Conclusion
The inclusion of the NNRTI efavirenz in once daily ART for HIV patients with a history of IDU receiving methadone maintenance results in a significant reduction in methadone plasma concentrations mediated by enzyme induction. It is important to distinguish efavirenz neurological effects which were observed between days 1–5 of therapy from symptoms of methadone withdrawal which occurred from day 8 onwards. The dose of methadone was adjusted by increments of 10 mg to counteract the efavirenz inducing effect.
Keywords: efavirenz, HIV, methadone
Introduction
Injection drug use is the second most common risk factor for the acquisition of Human Immunodeficiency Virus (HIV) [1]. Many patients are reluctant to accept treatment for their HIV, and physicians are generally unwilling to prescribe antiretroviral therapy (ART) until their drug habit has stabilized [2, 3]. Linking antiretroviral therapy with daily directly observed methadone maintenance therapy is an attractive approach in an attempt to resolve this problem. The current standard of antiretroviral therapy includes a three drug combination consisting of two nucleoside analogues in addition to a protease inhibitor or a non-nucleoside reverse transcriptase inhibitor (NNRTIs) [4]. The convenient once daily dosing of the NNRTIs makes them an attractive option for directly administered therapy. The potential for an interaction between NNRTIs and methadone has been described with reports of methadone withdrawal symptoms in patients receiving nevirapine (NVP) [5, 6]. To date there have been no definitive tolerability studies of efavirenz (EFV) in injection drug users, or pharmacokinetic studies to assess the interaction between methadone and EFV. We designed a study to assess the effect of EFV on methadone pharmacokinetics, the timing of withdrawal symptoms, and the requirement for a dose escalation in methadone. Based on these results we have produced recommendations for the prescribing of EFV to injection drug users concurrently receiving methadone.
Methods
Patients attending the drug treatment clinic on stable methadone maintenance therapy, who fulfilled standard criteria to commence ART, were recruited into the study. The local ethics committee approved the study, and the subjects consented to the study after full explanation of what was involved. Patients were aware that they were commencing medications that possibly would interfere with their methadone. They were not aware that the effect might induce withdrawal symptoms. Eleven patients (four females, seven males) participated in the study. All patients were regularly attending the drug treatment clinic for at least a period of 3 months, receiving daily observed methadone maintenance therapy (dose 35–100 mg daily, median 80 mg). All patients received a single dose of methadone that prevented the onset of withdrawal symptoms in between doses. The mean age of patients participating in the study was 35 years (range 24–45 years). The mean CD4 count at baseline was 172 × 106 l−1 (range 10–900 × 106 l−1), and HIV PCR RNA was 145 079 copies/ml (range 1118–500 000 copies/ml; Roche Ultrasensitive Assay®). All patients commenced oral triple antiretroviral therapy that included efavirenz (600 mg once daily) and two nucleoside analogues (4×stavudine and didanosine; 4×zidovudine and lamivudine; 2×stavudine and lamivudine; 1×stavudine and abacavir). There is no evidence that any of these nucleoside ART drugs interact with CYP3A4 [9].
Any neurological symptoms were carefully observed and recorded throughout the study period. At each clinic visit supervised urine samples were obtained for toxicology screening, and patients were assessed for evidence of methadone withdrawal., i.e. perspiration, agitation, sneezing, diarrhoea, leg cramps, pupillary diameter, rhinorrhea, yawning. None of the patients was coprescribed any additional drugs that would be expected to interfere with the metabolism of methadone, e.g. rifampicin, phenytoin, barbiturates, carbamazepine, fluconazole.
There were 2 study days where the pharmacokinetics of methadone were determined alone (day 1), or in the presence of antiretroviral therapy (day 2). Following three standardized days of directly observed methadone in their drug treatment clinic (DTC), patients attended the day ward at 08.30 h on study day 1, where an intravenous cannula was inserted to facilitate blood sampling. The patients were then administered their methadone under supervision, and blood samples for methadone analyses were taken at times 0, 1, 2, 3, 4, 5, 6, 7, 8, 24 h post dosing. These samples were centrifuged without delay and the separated plasma was heated to 58 °C for 30 min to inactivate HIV. Plasma was stored at −70 °C until drug analysis using high performance liquid chromatography (h.p.l.c.). Patients then commenced their antiretroviral therapy that included two nucleosides plus EFV 600 mg once daily. On the morning of day 14 or 21 (depending on the timing of withdrawal symptoms), they reattended the day ward for a repeat pharmacokinetic profile. The procedure was identical to day 1 except for the addition of their antiretroviral therapy (including EFV) to the morning medication. Any adjustment in methadone dose did not occur until after the second PK study day.
Methadone assay
Plasma samples in duplicate (0.25 ml) were pipetted into glass tubes, 25 µl internal standard (trihexyphenidyl HCl; 5 µg ml−1) and 250 µl K2CO3 (2 m) were added. Tube contents were mixed thoroughly. Standard curves were prepared containing blank plasma and methadone at a concentration range of 20–800 ng ml−1. Quality control samples for the assessment of precision and accuracy of the assay were prepared by adding known quantities of methadone to blank plasma samples. Test samples, standards and quality control samples were extracted with hexane (3 ml) for 10 min. After centrifuging for 5 min at 4000 g the organic phase was transferred to clean tubes and evaporated to dryness. Extracts were reconstituted into the h.p.l.c. mobile phase (0.1 ml) and transferred to vials for injection into the h.p.l.c.
Methadone and the internal standard were resolved on a Luna 5C8 column (5µ; 150 × 4.6 mm; Phenomenex, Macclesfield, UK) with a mobile phase of 25 mm sodium phosphate buffer pH 2.5: acetonitrile for u.v. (+ 0.15% TEA) (69:31) at a flow rate of 1 ml min−1. Absorbance was monitored at 210 nm. Peaks of interest, methadone (retention time = 14 min) and internal standard (retention time = 10.5 min) were quantified using a Kontron MT2 data acquisition system.
The limit of quantification was 2 ng ml−1. Inter-assay variability was determined with three different control samples containing nominal concentrations of 50, 200 and 400 ng ml−1. The coefficients of variation were 9.9%, 4.81% and 4.19%, respectively (n = 6). Intra-assay precision was determined with samples containing 50 and 400 ng ml−1. The coefficients of variation were 3.18% and 1.33%, respectively (n = 6).
Pharmacokinetic and statistical analysis
The pharmacokinetic parameters determined for methadone included the maximum plasma concentration (Cmax) and the area under the concentration time curve to 24 h (AUC(0–24 h)). Cmax was obtained by inspection of the data. AUC values were determined by noncompartmental analysis using TOPFIT computer software (Gustav Fischer Verlag, Stuttgart, Germany) [7]. Differences in pharmacokinetic parameters were compared using the paired Student's t-test. A P value of < 0.05 was considered statistically significant.
Results
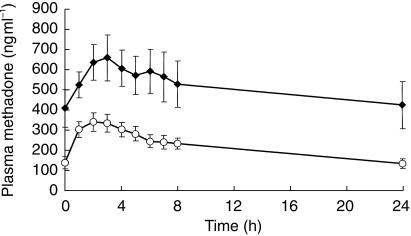
When EFV was combined with methadone, there was a marked decrease in the mean Cmax of methadone from 689 (range 212–1568) to 358 (205–706) ng ml−1, P = 0.007:95% CI 112–549 (Figure 1). The mean AUC(0,24 h) for methadone also significantly reduced from 12341 (range 3682–34147) to 5309 (2430–10349) ng ml−1 h in the presence of EFV, P = 0.012:95% CI 1921–12143.
Figure 1.
Plasma methadone concentrations alone (♦) and in combination (○) with 600 mg efavirenz. Results are mean±s.e. mean from 11 patients.
Five of 11 patients (45%) reported mild to moderate symptoms of dizziness, agitation, and restlessness from day 1–5 that resolved spontaneously over the subsequent week. Nine of 11 patients complained of symptoms consistent with methadone withdrawal from day 8 to 10 onwards. They were clinically evaluated to assess the extent of their symptoms, and received an increase in methadone dose initially in increments of 10 mg until their symptoms resolved. Methadone doses were not altered until after the second pharmacokinetic study day. The mean increase in methadone dose required was 22% (range 15–30 mg).
Discussion
Patients with HIV disease will receive multiple and prolonged dosing regimens not only for the treatment of HIV disease, but for the treatment and prophylaxis of opportunistic infections [8]. The standard of care for antiretroviral therapy involves using a triple combination of antiretrovirals, usually consisting of two nucleoside analogue reverse transcriptase inhibitors, and either a protease inhibitor or a non-nucleoside reverse transcriptase inhibitor [4]. The issue of drug–drug interactions and adherence with therapy arises as one of the major problems associated with current therapy [9]. Compared with other risk groups, injection drug users are twice as likely not to be receiving highly active antiretroviral therapy (HAART), and this increases to three times if they are not enrolled in a drug treatment programme [2]. Only 40% of injection drug users eligible for antiretroviral therapy are receiving it, and only 37% of this group are adherent with therapy [3]. The optimal way to manage HIV infection in injection drug users is to link antiretroviral therapy with stable methadone maintenance therapy. Administration of once daily antiretroviral therapy in conjunction with directly observed methadone maintenance therapy, will optimize adherence with drug treatment.
There have been few studies of the potential interactions between methadone and antiretroviral drugs. However, knowledge of the pharmacokinetics of methadone facilitates prediction of potential interactions. Methadone is extensively metabolized by the cytochrome P450 hepatic enzyme system, yielding an N-demethylated metabolite that cyclizes spontaneously into 2-ethylidene-1,5-dimethyl-3,3-diphenylpyrrolidine [10]. While cytochrome 450 (CYP) 2C8, 2C18, and 2D6 isoenzymes have a role in the demethylation of methadone, the CYP3A4 isoenzyme has been shown to be the major enzyme involved [10]. Many metabolites have been traced and identified in human urine and they are free from pharmacological effect [11].
It has been previously demonstrated that drugs which are inhibitors or inducers of CYP450 may alter the metabolism of methadone, producing symptoms of narcotic overdose (inhibition), or precipitating symptoms of methadone withdrawal (induction) when given to patients on stable MMT [12–18]. For patients on MMT receiving concurrent fluconazole, an inhibitor of CYP450, a mean increase in the systemic methadone exposure of 34.8% has been demonstrated. However, no signs or symptoms of methadone overdose were demonstrated during the study, and no patients complained of methadone withdrawal during the post study period [12]. Phenytoin and carbamazepine are both inducers of the CYP450 enzyme system and there have been reports of methadone withdrawal when coadministered [13, 14]. Injection drug users are at an increased risk of acquiring tuberculosis and the association between rifampicin and reduced effectiveness of methadone has been well described [15–18]. In a cohort of 30 patients receiving methadone and rifampicin, 70% of patients required an increase in their methadone dose, with the onset of withdrawal symptoms from 1 to 33 days after the initiation of therapy [15]. There have been other similar reports of this interaction, and the requirement for additional methadone in such patients [16–18].
Nevirapine, a non-nucleoside reverse transcriptase inhibitor, is a potent inducer of CYP450 3A4 [19]. There have been no pharmacokinetic studies to date demonstrating induction of methadone metabolism when NVP is given to patients on methadone maintenance therapy. Symptoms of methadone withdrawal have been reported in seven patients 4–8 days after initiating therapy with NVP [5]. This was a retrospective review and reductions in mean trough methadone levels were only available for two patients. Otero et al. have also described a series of four patients experiencing methadone withdrawal 6–15 days after commencing NVP therapy which required a 33–100% increase in methadone dosage [6].
Efavirenz is an inducer primarily of CYP3A4, and to a lesser degree CYP2B6. In vitro it may inhibit CYP3A4, 2C9, and 2C19 [20]. Anecdotal reports suggest that it may precipitate symptoms of methadone withdrawal when these drugs are given concurrently [5, 6]. In the present study, nine patients complained of symptoms of methadone withdrawal. The mean time for symptom onset was 8–10 days. This is consistent with previous studies showing maximum rifampicin induced changes in the hepatic drug metabolizing enzymes within 9–12 days of initiating therapy [21]. However the time-scale for symptom onset is longer than that seen in previous reports of NVP induced methadone withdrawal, where symptoms began as early as day 4 onwards. Following clinical evaluation to assess the severity of their withdrawal symptoms, the methadone dose was increased initially in 10 mg increments. The mean increase in dose required was 16 mg (range 15–30 mg). The mean increase in methadone dose of 22% was significantly lower than would have been predicted from the mean 60% reduction in AUC(0,24 h) for methadone over the initial 2–3-week period. This raised the possibility that during the period of EFV induced enzyme induction, there is concurrent detoxification from methadone. Alternatively if the dose of methadone was considerably above that required to prevent withdrawal then a decrease in concentration would not result in severe withdrawal symptoms. Methadone has been associated with significant drug toxicity and mortality, especially during the initial period of introduction onto a methadone maintenance programme [22, 23]. It is important therefore not to overestimate both how much extra methadone is required by the patient, and how early in treatment such an increment will be required.
Neurological symptoms of moderate to severe intensity have been described in up to one quarter of patients receiving EFV [20]. These include dizziness, headache, insomnia, impaired concentration, vivid dreams, nightmares, and agitation. Symptoms usually begin during the first 1–2 days of therapy, and generally resolve after the first 2–4 weeks. Dosing at bedtime seems to improve the tolerability of these symptoms. In this study, EFV was administered daily with directly observed methadone maintenance therapy, in the morning, in order to achieve 100% adherence with therapy. In our cohort, 5 of 11 patients complained of mild to moderate neurological symptoms. They were not consistent with symptoms of methadone withdrawal and in all cases began between day 1–5 and resolved spontaneously by day 14. While early (day 1–5) neurological symptoms may appear to mimic symptoms of methadone withdrawal, a careful evaluation and clinical assessment of the patient should differentiate between EFV toxicity and methadone withdrawal.
The consequences of removing the enzyme-inducing agent must also be remembered. Whether a drug has a high or low hepatic clearance, on chronic oral dosing, the determinants of the steady state plasma concentration are unbound drug fraction and the intrinsic clearance. Enzyme induction would result in an increase in intrinsic clearance which would result in an increased clearance for low hepatic clearance drugs, e.g. methadone but reduced bioavailability of drugs with high hepatic clearance [24, 25]. On removing the enzyme-inducing agent, the effects of induction will gradually diminish over the subsequent 2–3 weeks [21]. Therefore, withdrawal of EFV therapy owing to either an adverse drug reaction, or treatment failure, could result in potentially toxic methadone levels if the dose is not reduced to pretreatment levels. In such circumstances, it is the policy within our unit to gradually reduce the dose of methadone over a period of 2–3 weeks to the pretreatment dose.
For patients commencing EFV whom are also receiving methadone maintenance therapy, we recommend frequent medical and psychiatric assessments and patients should attend daily for supervised methadone and antiretroviral therapy. Early (day 1–5) EFV-related neurological symptoms must be clinically differentiated from symptoms of methadone withdrawal, as such symptoms will resolve spontaneously. Symptoms of methadone withdrawal may be expected from day 7–10 onwards. During the initial 2–3 weeks of therapy the increase in methadone dose required is not as great as would be expected from an individual's pharmacokinetic data. Those patients requiring higher doses of methadone initially will require a greater increase in methadone dose with EFV therapy. Therefore, our current policy is that any increase in methadone dose must be done in increments of 10 mg, with daily supervision of dosing and clinical evaluation for signs of opioid withdrawal.
References
- 1.National Consensus Development Panel on effective medical treatment of opiate addiction. Effective medical treatment of opiate addiction. JAMA. 1998. pp. 22–30. [PubMed]
- 2.Strathdee SA, Palepu A, Cornelisse A, et al. Barriers to use of free antiretroviral therapy in injection drug users. JAMA. 1998;280:547–549. doi: 10.1001/jama.280.6.547. [DOI] [PubMed] [Google Scholar]
- 3.Celentano BD, Vlahov D, Cohn S, Shadle VM, Obasanjo O, Moore RD. Self reported antiretroviral therapy in injection drug users. JAMA. 1998;280:544–546. doi: 10.1001/jama.280.6.544. [DOI] [PubMed] [Google Scholar]
- 4.Carpenter CCJ, Fischl MA, Hammer SM, et al. Updated recommendations of the International AIDS Society-USA Panel – antiretroviral therapy for HIV infection in 1998. JAMA. 1998;280:78–86. doi: 10.1001/jama.280.1.78. [DOI] [PubMed] [Google Scholar]
- 5.Altice FL, Friedland GH, Cooney EL. Nevirapine induced opiate withdrawal among injection drug users with HIV infection receiving methadone. AIDS. 1999;13:957–962. doi: 10.1097/00002030-199905280-00012. [DOI] [PubMed] [Google Scholar]
- 6.Otero MJ, Fuertes A, Sanchez R, Luna G. Nevirapine induced withdrawal symptoms in HIV patients on methadone maintenance programmes -an alert. AIDS. 1999;13:1004–1005. doi: 10.1097/00002030-199905280-00025. [DOI] [PubMed] [Google Scholar]
- 7.Heinzel G, Woloszczak R, Thomann P. Topfit, Version 2: Pharmacokinetic and Pharmacodynamic Data Analysis System for the PC. Stuttgart: Gustav Fischer; 1993. [Google Scholar]
- 8.Fogelman I, Lim L, Bassett R, et al. Prevalence and patterns of concomitant medications among participants in 3 multicentre HIV type 1 clinical trials. J Acq Immune Def Syndrome. 1994;7:1057–1063. [PubMed] [Google Scholar]
- 9.Barry M, Mulcahy F, Merry C, Gibbons S, Back D. Pharmacokinetics and potential interactions amongst antiretroviral agents used to treat HIV infection. Clin Pharmacokinet. 1999;36:289–304. doi: 10.2165/00003088-199936040-00004. [DOI] [PubMed] [Google Scholar]
- 10.Iribarne C, Berthou F, Baird S, et al. Involvement of cytochrome P450 3A4 enzyme in the N-demethylation of methadone in human liver microsomes. Chem Res Toxicol. 1996;9:365–373. doi: 10.1021/tx950116m. [DOI] [PubMed] [Google Scholar]
- 11.Sullivan HK, Due SL, McMahon RE. Methadone N-oxide in the urine of methadone maintained subjects. J Pharm Pharmacol. 1973;25:1009–1010. doi: 10.1111/j.2042-7158.1973.tb09998.x. [DOI] [PubMed] [Google Scholar]
- 12.Cobb MN, Desai J, Brown LS, Zannikos PN, Rainey PM. The effects of fluconazole on the clinical pharmacokinetics of methadone. Clin Pharmacol Ther. 1998;63:655–662. doi: 10.1016/S0009-9236(98)90089-3. [DOI] [PubMed] [Google Scholar]
- 13.Tong TG, Pond SM, Kreek MJ. Phenytoin-induced methadone withdrawal. Ann Intern Med. 1981;94:349–351. doi: 10.7326/0003-4819-94-3-349. [DOI] [PubMed] [Google Scholar]
- 14.Saxon AJ, Whittacker S, Hawker S. Valproic acid-unlike other anticonvulsants has no effect on methadone metabolism: 2 cases. J Clin Psych. 1989;50:228–229. [PubMed] [Google Scholar]
- 15.Kreek MJ, Garfield JW, Gutjahr CL, Giusti LM. Rifampicin-induced methadone withdrawal. N Engl J Med. 1976;294:1104–1106. doi: 10.1056/NEJM197605132942008. [DOI] [PubMed] [Google Scholar]
- 16.Baciewicz AM, Self TH. Rifampin drug interactions. Arch Intern Med. 1984;144:1667–1671. doi: 10.1001/archinte.144.8.1667. [DOI] [PubMed] [Google Scholar]
- 17.Raistrick D, Hay A, Wolff K. Methadone maintenance and tuberculosis treatment. Br Med J. 1996;313:925–926. doi: 10.1136/bmj.313.7062.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Holmes VF. Rifampicin induced methadone in AIDS. J Clin Psychopharmacol. 1990;10:443–444. [PubMed] [Google Scholar]
- 19.Havlir D, Cheesman SH, McLaughlin M, et al. High dose nevirapine: safety, pharmacokinetics, and antiviral effect in patients with HIV infection. J Infect Dis. 1995;171:537–545. doi: 10.1093/infdis/171.3.537. [DOI] [PubMed] [Google Scholar]
- 20.Adkins JC, Noble S. Efavirenz. Drugs. 1998;56:1055–1064. doi: 10.2165/00003495-199856060-00014. [DOI] [PubMed] [Google Scholar]
- 21.Barry M, Feely J. Enzyme induction and inhibition. Pharmacol Ther. 1990;48:71–94. doi: 10.1016/0163-7258(90)90019-x. [DOI] [PubMed] [Google Scholar]
- 22.Drummer OH, Opeskin K, Syrjanan M, Cordner SM. Methadone toxicity causing death in 10 subjects starting on a methadone maintenance programme. Am J Forensic Med Pathol. 1992;13:346–350. doi: 10.1097/00000433-199212000-00017. [DOI] [PubMed] [Google Scholar]
- 23.Harding-Pink D. Methadone: one person's maintenance dose is another's poison. Lancet. 1993;341:665–666. doi: 10.1016/0140-6736(93)90427-i. [DOI] [PubMed] [Google Scholar]
- 24.Wilkinson GR, Shand DG. A physiological approach to hepatic drug clearance. Clin Pharmacol Ther. 1975;18:377–390. doi: 10.1002/cpt1975184377. [DOI] [PubMed] [Google Scholar]
- 25.Rowland M, Tozer TN. Integration with kinetics. In: Balado D, editor. Clinical Pharmacokinetics, Concepts and Applications. 3. Philadelphia: Williams & Wilkins; 1995. pp. 184–200. [Google Scholar]