Abstract
Aims
To determine the within-subject reproducibility of the forearm blood flow response to acetylcholine and the β2-adrenoceptor agonist albuterol as measured by strain gauge plethysmography. To examine the influence of strain gauge placement on these responses.
Methods
Vasodilator response to brachial artery infusion of drugs was assessed by strain gauge plethysmography in six healthy men on each of three occasions separated by 1 week. Strain gauges were placed on both arms at the point of maximum diameter. On the infused arm two further gauges were positioned approximately 4 cm proximal and distal to the middle gauge.
Results
Within-subject coefficients of variation (WCV) of absolute blood flow responses for each dose of acetylcholine (7.5, 15, 30 µg min−1) ranged from 24% to 27%, as compared with WCV values of 41% to 62% for the percentage changes in blood flow ratio (infused : noninfused arm). For albuterol (0.3, 1, 3 µg min−1) the corresponding WCV values were 16% to 19% and 30% to 55% for absolute blood flow and percentage change in blood flow ratio, respectively. WCV for the area under dose-response curve (AUC) for absolute blood flow was 18% and 13% for acetylcholine and albuterol, respectively. Vasodilator responses were similar whether recorded proximal to or at the point of maximal forearm circumference. Distal strain gauge misplacement underestimated responses and the difference was greater for acetylcholine than for albuterol.
Conclusions
In healthy men, the WCV for responses expressed as absolute blood flow, to acetylcholine and albuterol ranges from 16% to 27%.
Keywords: acetylcholine, albuterol, endothelium, reproducibility, strain-gauge plethysmography
Introduction
Forearm vasodilator responses, measured using strain gauge plethysmography, to brachial artery infusion of endothelium-dependent vasodilators such as acetylcholine have been used to demonstrate endothelial dysfunction in conditions associated with atherosclerosis [1]. With the recognition that, in many conditions, endothelial dysfunction is reversible such tests are being increasingly used to assess effects of interventions on endothelial function [1]. The power of such studies depends on the within-subject reproducibility of vasodilator responsiveness assessed by strain gauge plethysmography. This has not, however, been assessed formally. In addition, the optimal method for expressing forearm vasodilator response has not been established. The blood flow response is usually expressed as the absolute value of forearm blood flow during drug infusion [2–5], or the percentage change in blood flow ratio in the infused : noninfused arm [6–8]. When interventions are assessed comparisons of the blood flow responses to each dose of agonist are usually made using repeated measures analysis of variance [2–5] or a summary statistic such as the area under the dose-response curve (AUC) [7, 8] is used.
The primary objectives of the present study were to determine (1) within-subject variability of responses to the endothelium-dependent vasodilators acetylcholine [9] and albuterol (salbutamol) [10, 11] and (2) the method for expressing responses which minimizes such variability. Albuterol is more stable than acetylcholine (which is rapidly broken down by cholinesterases [12, 13]) suggesting that forearm blood flow responses to albuterol may be more reproducible than those to acetylcholine. Subsidiary aims were to examine the influence of the position of strain gauge placement on the arm and the intrinsic reproducibility of the strain gauges (as assessed off the limb).
Methods
Six healthy nonsmoking men aged 22–30 years participated in the study. No subject was taking drug therapy, all were normotensive, with total cholesterol < 6.0 mmol l−1. The study was approved by the St Thomas’ Hospital Research Ethics Committee and all subjects gave written informed consent. Subjects attended at the same time of the day on three occasions separated by at least 1 week, and were asked to maintain their usual diet. Forearm blood flow measurements were performed in a quiet clinical laboratory (temperature controlled to between 24 and 26 °C during each study). Blood flow was measured in both forearms using venous occlusion plethysmography with strain gauges [14], electrically calibrated [15]. Cuffs were applied to both upper arms and wrists. Venous (50 mmHg) and arterial (180 mmHg) occlusions were obtained using Hokanson E20 rapid cuff inflators. Strain gauges (Hokanson, Bellevue, US) were placed at the point of maximal forearm circumference. Additional strain gauges were placed on the left arm (infused arm) at positions 4 cm proximal and 4 cm distal to this point. The distance of the gauges from the point of insertion of the needle on the left arm and from the medial epicondyle on the right arm were measured and kept constant at each visit. The same gauges were applied to the same position and connected to the same transducer on each occasion. Output of the strain gauge transducers was digitized by a MacLab 400 (AD Instruments, Australia) analogue to digital converter and displayed on a monitor. An unmounted 27 gauge steel needle (Cooperís Needleworks, Birmingham, UK) was sealed by dental wax to an epidural catheter (Portex, Hythe, UK) and inserted into the left brachial artery under sterile conditions using < 1 ml of 1% lignocaine hydrochloride (Antigen Pharmaceuticals, Rosecrea, Ireland) to provide local anaesthesia. Saline (0.9% NaCl) or drugs (acetylcholine chloride, CIBA Vision Ophthalmics, Southampton, UK; albuterol sulphate, Allen and Hanburys, Middlesex, UK) dissolved in saline were infused at 1.0 ml min−1 by constant-rate infusion pump (IVAC P3000, Welmed, Hampshire, UK).
Basal blood flow was recorded after a 15-min infusion of saline. Three cumulative doses of acetylcholine (7.5, 15, 30 µg min−1, each dose for 6 min) were infused followed by saline for 18 min to allow blood flow to return to baseline. After baseline measurements, a cumulative infusion of albuterol (0.3, 1, 3 µg min−1, each dose for 6 min) was given. Forearm blood flow was recorded during the last 3 min of each infusion period using 10 s venous occlusions every 15 s. Blood flow was calculated from the last 5 venous inflations using the MacLab chart software (v3.5, AD Instruments, Australia) in units of ml min−1 per 100 ml of forearm volume [14]. Blood pressure was measured by mercury sphygmomanometry at the end of the study.
Each strain gauge was electrically calibrated [15] following each study. In addition, gauges were mechanically calibrated off the limb using a precision calibration device (C and T Technical Services, Gillingham, UK) which produced incremental changes in gauge circumference.
Analysis
Forearm blood flow (Q) responses to vasodilator agonists were expressed as absolute blood flow in the infused arm (i) during drug (d) infusion (Qid), and as the percentage increase in ratio of blood flow in the infused to noninfused (ni) arm during infusion of saline (s) or drug according to the formula proposed by Benjamin et al. [16]:
| 1 |
Absolute blood flow in the infused arm during drug infusion was also corrected by subtracting any increase in flow in the noninfused arm occurring between the time of drug infusion and that of saline infusion. This correction factor was adjusted to take into account the ratio of flows at the outset during infusion of saline:
| 2 |
Within-subject variation in responses was quantified using the within-subject coefficient of variation (WCV). WCV provides a measure of the within-subject standard deviation as a percentage of the mean of the measurements. Within-and between-subject variances were approximately proportional to the mean blood flow responses suggesting that the blood flow data were approximately log-normally distributed. WCV were thus determined using the maximum likelihood estimator for log normal data developed by Quan & Shih [17]. This was done for each method of expressing the blood flow response, for each dose and for the area under the dose-response curve (AUC). Differences between WCV for each method were evaluated by comparing within-subject variances of log-transformed blood flow responses [18]. P < 0.05 was considered significant.
Results
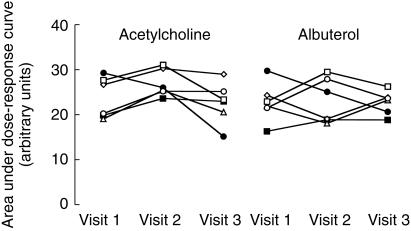
Electrical calibration of the strain gauges remained stable with between-visit variations less than 0.4%. Variation in mechanical calibration between-visits was less than 4%. Mean (for all subjects and all visits) values of forearm blood flow derived from the middle strain gauge on the infused arm, and percentage change in forearm blood flow, derived from this reference gauge on the infused arm and the strain gauge on the noninfused arm, are tabulated in Table 1 together with WCV. WCV for absolute blood flow responses (Qid) to the three doses of acetylcholine ranged from 24% to 27% and were significantly less than corresponding values for the percentage change in forearm blood flow ratio (41% to 62%, P < 0.05). WCV for the AUC calculated from absolute blood flow responses to acetylcholine was also significantly lower than the value for the percentage change in blood flow ratio (18% vs 38%, P < 0.05). WCV for Qid to albuterol were also less than the percentage change in blood flow ratio (absolute blood flow: 16% to 19% vs forearm blood flow ratio: 30% to 55%), but the difference did not reach statistical significance (P = 0.1). Correction of absolute blood flow by subtracting absolute changes in the noninfused arm (equation 2) had no significant effect on the reproducibility of blood responses to acetylcholine or albuterol (WCV for the AUC calculated from corrected absolute blood flow responses to acetylcholine 18% vs 18%, P = NS for uncorrected blood flow and that for albuterol 15.0% vs 13%, P = NS). Expressing responses as the increase in absolute blood flow above baseline (Qid-Qis) did not improve reproducibility (WCV for the AUC for acetylcholine using Qid-Qis 21% vs 18% using Qid, P = NS and that for albuterol 17% vs 13%, P = NS). Variation in the responses to acetylcholine and albuterol expressed as the AUC for absolute blood flow, the measure showing least variability over the three visits, is shown in Figure 1.
Table 1.
Mean forearm blood flow (FBF) and percentage change in forearm blood flow ratio (ΔFBFR) and within-subject coefficients of variation (WCV).
| Drug | Dose (µg min−1) | FBF (ml min−1 100 ml−1) | FBF WCV (%) | ΔFBFR (%) | ΔFBFR WCV (%) |
|---|---|---|---|---|---|
| ACh | 7.5 | 8.9 | 24 | 150 | 45† |
| ACh | 15 | 10.8 | 26 | 168 | 62† |
| ACh | 30 | 17.2 | 27 | 413 | 41† |
| ACh | AUC | 24.5⋆ | 18 | 600⋆ | 38† |
| ALB | 0.3 | 7.4 | 19 | 102 | 55 |
| ALB | 1.0 | 10.9 | 17 | 176 | 30 |
| ALB | 3.0 | 14.9 | 16 | 232 | 48 |
| ALB | AUC | 23.1⋆ | 13 | 340⋆ | 25 |
ACh: acetylcholine; ALB: albuterol; AUC: area under dose response curve.
Arbitrary units.
Significantly greater than FBF WCV.
Figure 1.
Area under the absolute blood flow dose–response curve for acetylcholine and albuterol determined on each of three visits. This measure of the response to the vasodilator drugs showed least variability with within-subject coefficients of variation of 18 and 13% for acetylcholine and albuterol, respectively. ○ subject 1; □ subject 2; ▵ subject 3; ⋄ subject 4; • subject 5; ▪ subject 6.
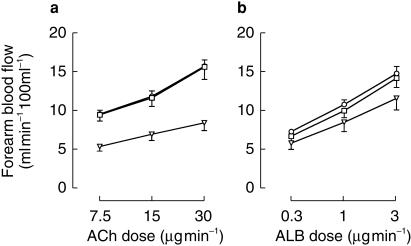
The effect of strain gauge placement on blood flow responses to acetylcholine and albuterol is shown in Figure 2. There was no significant difference between blood flows derived from the proximal and middle gauges but those derived from the distal gauge were lower than those derived from the middle gauge for both acetylcholine and albuterol (P < 0.01). The difference between blood flows derived from the middle and distal gauges was greater for acetylcholine than for albuterol (P < 0.05).
Figure 2.
Forearm blood flow (mean±s.e. mean for all subjects) recorded from strain gauges placed at the point of maximal forearm circumference (circles) and 4 cm proximal (squares) and distal (triangles) to this point. (a) Blood flow during infusion of acetylcholine (ACh). (b) Blood flow during infusion of albuterol (ALB).
Discussion
The present study is the first to provide an estimate of within-subject reproducibility of the response to acetylcholine and thus allow an estimate of the power of forearm blood flow methods to detect changes in endothelial function. The term reproducibility refers to the similarity of results obtained when measurements are repeated under conditions where factors such as physiological variation within subjects in addition to the measuring device may contribute to variability of the measurement [19]. Variability due to the strain gauge plethysmograph itself was assessed using a mechanical calibrator and was found to be low in comparison with the overall variability. The measurement of reproducibility is subject to some controversy. We have used the WCV. This is preferred over the intraclass correlation coefficient since this is less easy to interpret than the WCV and influenced by the relative heterogeneity of the study group [17]. The WCV is appropriate when the measurement scale bears intrinsic meaning [17] as is the case when measurements are made in units of ml min−1 per 100 ml of forearm volume.
The method used to express the vasodilator response influences within-subject reproducibility. Vasodilator blood flow responses may be influenced by changes in basal blood flow caused, for example, by altered state of arousal. To control for this it has been suggested that blood flow responses are expressed as the change in ratio of blood flow in the infused arm to that in the noninfused arm [20]. An assumption implicit in the use of this ratio is that flow in the infused arm will be influenced by a change in basal flow to the same degree as flow in the noninfused arm. This assumption is likely to hold to a close approximation during the use of vasoconstrictor drugs or vasodilator drugs producing relatively small changes in blood flow. Large changes in blood flow in the infused arm are, in contrast, unlikely to be affected by changes in basal flow to the same degree as those in the contralateral noninfused arm. For example, a change in state of arousal which causes a 50% increase of 1 ml min−1 100 ml−1 (from a basal level, say, 2 ml min−1 100 ml−1) in blood flow in the noninfused arm, for example, will not cause a 50% increase in flow of 10 ml min−1 100 ml−1 during acetylcholine infusion (from a value, say, 20 ml min−1 100 ml−1) in the infused arm. In this case use of the ratio method will, far from controlling for changes in state of arousal, introduce spurious changes in the response. In the present study we found that vasodilator responses to doses of acetylcholine that are commonly used to assess endothelial function were significantly less variable when expressed as absolute blood flows rather than as a percentage increase in blood flow ratio. An attempt to correct absolute blood flow by subtracting absolute changes in the noninfused arm (on the assumption that changes due to arousal should be the same in both arms) did not reduce variability. This suggests that the main source of variability in blood flow in the infused arm during studies of this kind is not due to changes in arousal or other factors that affect basal blood flow but rather to variability in the vasodilator response itself.
Our finding that blood flow responses are most reproducible when expressed as absolute blood flow is not at variance with that of Petrie et al. [21] who found that vasoconstrictor responses were less variable when expressed as percentage change in blood flow ratio but that a vasodilator response (to forearm exercise) was less variable when expressed as absolute blood flow. This is plausible since vasoconstrictor drugs produce relatively small absolute changes in blood flow which may be affected by changes in state of arousal to a similar degree as flow in the noninfused arm. We also found that absolute blood flow tended to be more reproducible than the increase in flow above baseline. This method of expressing the response may be appropriate, however, when studying the response to an agonist in the presence of an antagonist (for example l-NMMA) which produces a change in basal blood flow [10]. In this case the influence of changes in basal flow needs to be examined by the use of appropriate control agonists.
Although not influencing within subject variability, a further source of variation in the presentation of forearm blood flow results relates to the quantification of drug dose. This is usually expressed as mass infused into the brachial artery per unit time. Chin-Dusting and colleagues have recently proposed that dose of drug should be corrected according to the local volume of distribution [22]. Such a correction, however, neglects effects of blood flow and destruction of the agonist within conduit vessels. In the case of acetylcholine this is a major factor influencing the response [13, 23]. Thus although forearm dimensions need to be taken into account when making comparisons across groups, correction of the dose for forearm volume adds unjustified complexity.
A subsidary aim of our study was to address the influence of strain gauge placement. Whitney has shown that when measuring blood flow responses to exercise similar results are obtained from strain gauges placed at different positions along the mid-forearm [14]. In the case of acetylcholine which, as discussed above, is highly unstable in blood, dilation of arteriovenous communications and venous swelling may be distributed to the proximal forearm (due to valves preventing venous backflow to the distal forearm). The forearm blood flow response to acetylcholine per 100 ml forearm tissue may therefore vary with strain gauge distance from the point of infusion, decreasing with distal misplacement. In the present study we examined such variation by placing strain gauges at increasing distances from the point of infusion. As expected the response to acetylcholine decreased with increasing strain gauge distance from the infusion cannula (P < 0.01). However, within the range of positions used in common practice, blood flow responses were similar and WCV did not differ significantly irrespective of whether responses were calculated from the first or second strain gauges or the mean of these. Thus for most purposes use of a single strain gauge is satisfactory provided its distance from the point of infusion is standardized. Use of two gauges has the advantage of providing a within subject check on strain gauge reliability during the course of a study. The dependence of the blood flow response on strain gauge position was significantly greater for acetylcholine than for albuterol. This is consistent with the rapid destruction of acetylcholine.
In conclusion, the main findings of the present study are that, in determining the forearm blood flow response to acetylcholine, the position of strain gauge placement is not critical provided the gauge is placed at or proximal (but not distal) to the point of maximum forearm diameter. Within-subject variability is least when blood flow is expressed as the absolute flow during drug infusion. The WCV for the response to acetylcholine over the dose range 7.5–30 µg min−1 was 24% to 27% and, for the AUC, 18%. This means that, in healthy men, n = 20, a change in AUC response to acetylcholine of less than 20% can be detected with more than 90% power for differences considered significant at P < 0.05. Where responses to acetylcholine in groups with cardiovascular risk factors associated with endothelial dysfunction are blunted by more than 20% as is commonly observed [2–5, 7], such power should be sufficient to detect effects of interventions.
References
- 1.Anderson TJ. Assessment and treatment of endothelial dysfunction in humans. J Am Coll Cardiol. 1999;34:225–229. doi: 10.1016/s0735-1097(99)00259-4. [DOI] [PubMed] [Google Scholar]
- 2.Creager MA, Cooke JP, Mendelsohn ME, et al. Impaired vasodilation of forearm resistance vessels in hypercholesterolaemic humans. J Clin Invest. 1990;86:228–234. doi: 10.1172/JCI114688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chowienczyk PJ, Watts GF, Cockcroft JR, Ritter JM. Impaired endothelium-dependent vasodilation of forearm resistance vessels in hypercholesterolaemia. Lancet. 1992;340:1430–1432. doi: 10.1016/0140-6736(92)92621-l. [DOI] [PubMed] [Google Scholar]
- 4.McVeigh GE, Brennan GM, Johnston GD, et al. Impaired endothelium-dependent and independent vasodilation in patients with Type 2 (non-insulin-dependent) diabetes mellitus. Diabetologia. 1992;35:771–776. doi: 10.1007/BF00429099. [DOI] [PubMed] [Google Scholar]
- 5.Williams SB, Cusco JA, Roddy MA, Johnstone MT, Creager MA. Impaired nitric oxide-mediated vasodilation in patients with non-insulin-dependent diabetes mellitus. J Am Coll Cardiol. 1996;27:567–574. doi: 10.1016/0735-1097(95)00522-6. [DOI] [PubMed] [Google Scholar]
- 6.Stroes ESG, Koomans HA, de Bruin TWA, Rabelink TJ. Vascular function in the forearm of hypercholesterolaemic patients off and on lipid-lowering medication. Lancet. 1995;346:467–471. doi: 10.1016/s0140-6736(95)91322-x. [DOI] [PubMed] [Google Scholar]
- 7.Watts GF, O'Brien SF, Silvester W, Millar JA. Impaired endothelium-dependent and independent dilation of forearm resistance arteries in men with diet treated non-insulin dependent diabetes; role of dyslipidaemia. Clin Sci. 1996;91:567–573. doi: 10.1042/cs0910567. [DOI] [PubMed] [Google Scholar]
- 8.Calver A, Collier J, Vallance P. Inhibition and stimulation of nitric oxide in the human forearm bed of patients with insulin-dependent diabetes. J Clin Invest. 1992;90:2448–2554. doi: 10.1172/JCI116149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vallance P, Collier J, Moncada S. Effects of endothelium-derived nitric oxide on peripheral arteriolar tone in man. Lancet. 1989;ii:997–1000. doi: 10.1016/s0140-6736(89)91013-1. [DOI] [PubMed] [Google Scholar]
- 10.Dawes M, Chowienczyk PJ, Ritter JM. Effects of inhibition of the l-arginine/nitric oxide pathway on vasodilation caused by beta-adrenergic agonists in human forearm. Circulation. 1997;95:2293–2297. doi: 10.1161/01.cir.95.9.2293. [DOI] [PubMed] [Google Scholar]
- 11.Cardillo C, Kilcoyne CM, Quyyumi AA, Cannon Ro, III, Panza JA. Decreased vasodilator response to isoproterenol during nitric oxide inhibition in humans. Hypertension. 1997;30:918–921. doi: 10.1161/01.hyp.30.4.918. [DOI] [PubMed] [Google Scholar]
- 12.Duff F, Greenfield ADM, Shepherd JT, Thompson ID. A quantitative study of the response to acetylcholine and histamine of the blood vessels of the human hand and forearm. J Physiol (Lond) 1953;120:160–170. doi: 10.1113/jphysiol.1953.sp004883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chowienczyk PJ, Cockcroft JR, Ritter JM. Inhibition of acetylcholinesterase selectively potentiates NG-monomethyl-l-arginine resistant actions of acetylcholine in human forearm vasculature. Clin Sci. 1995;88:111–117. doi: 10.1042/cs0880111. [DOI] [PubMed] [Google Scholar]
- 14.Whitney RJ. The measurement of volume changes in human limbs. J Physiol (Lond) 1953;121:1–27. doi: 10.1113/jphysiol.1953.sp004926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hokanson DE, Sumner DS, Strandness De., Jr An electrically calibrated plethysmograph for direct measurement of limb blood flow. IEEE Trans Biomed Eng. 1975;22:25–29. doi: 10.1109/tbme.1975.324535. [DOI] [PubMed] [Google Scholar]
- 16.Benjamin N, Cockcroft JR, Collier JG, Dollery CT, Ritter JM, Webb DJ. Local inhibition of converting enzyme and vascular responses to angiotensin and bradykinin in the human forearm. J Physiol (Lond) 1989;412:543–555. doi: 10.1113/jphysiol.1989.sp017630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Quan H, Shih WJ. Assessing reproducibility by the within-subject coefficient of variation with random effects model. Biometrics. 1996;52:1195–1203. [PubMed] [Google Scholar]
- 18.Zar JH. Biostatistical analysis. 2. New Jersey: Prentice Hall; 1984. p. 125. [Google Scholar]
- 19.Dunn G. Design and analysis of reliability studies: the statistical evaluation of measurement errors. New York: Oxford University Press; 1989. [Google Scholar]
- 20.Benjamin N, Calver A, Collier J, Robinson B, Vallance P, Webb DJ. Measuring forearm blood flow and interpreting the response to drugs and mediators. Hypertension. 1995;25:918–923. doi: 10.1161/01.hyp.25.5.918. [DOI] [PubMed] [Google Scholar]
- 21.Petrie JR, Ueda S, Morris AD, Murray LS, Elliott HL, Connell JMC. How reproducible is bilateral forearm plethysmography ? Br J Clin Pharmacol. 1998;45:131–139. doi: 10.1046/j.1365-2125.1998.00656.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chin-Dusting JPF, Cameron JD, Dart AM, Jennings GLR. Human forearm venous occlusion plethysmography: methodology, presentation and analysis. Clin Sci. 1999;96:439–440. [PubMed] [Google Scholar]
- 23.Chowienczyk PJ, Cockcroft JR, Ritter JM. Blood flow responses to intra-arterial acetylcholine in man: effects of basal flow and conduit vessel length. Clin Sci. 1994;87:45–51. doi: 10.1042/cs0870045. [DOI] [PubMed] [Google Scholar]