Abstract
Drug treatment of depressive illness in the elderly differs from that in younger patients and there is no clear consensus as to first line treatment in the former. Nor is it possible to extrapolate directly from studies in younger patients to the elderly with these agents. Whilst there are over two dozen antidepressants currently marketed in the U.K., most studies have been on younger adults and have excluded very old and frail patients. Design short-comings of the few trials conducted in elderly patients do not allow accurate interpretation of differences in efficacy or safety between drugs.
This paper identifies key deficiencies in the evidence currently available in support of both older and newer antidepressant agents and makes the proposal that specific studies are required in the elderly to determine the efficacy and safety of antidepressants in the treatment of depressive illness. It outlines a Phase II and III clinical trial programme which could be used to provide adequate evidence of efficacy and safety of new agents and which conforms to current European guidelines. Dose finding studies, short-term efficacy, prevention of relapse (continuation therapy) and prevention of recurrence (maintenance therapy) studies are discussed as are key issues to be addressed in the trial protocol.
Keywords: antidepressants, elderly
Introduction
Depressive illness is the fourth leading cause of mortality and morbidity and is predicted to become the second leading cause by the year 2020, primarily as a result of the ageing population. Approximately 10% of people over the age of 65 years suffer from depression of sufficient severity to warrant intervention [1]. However, relatively few depressed older adults receive therapeutic interventions, despite suffering from an illness, which often runs a chronic or recurrent course and can be treated effectively [2].
Depression in later life differs from depression in younger subjects in aetiology, presentation, treatment and outcome [3]. The depressive syndrome needs to be viewed in the context of the associated ageing process and concomitant physical illness. Several different treatment modalities can be identified but pharmacotherapy remains a leading area.
The pharmacotherapy of depression in the elderly differs from that in younger patients in two major ways. It is widely acknowledged that older patients are more prone to the side-effects of antidepressant drugs and experience greater difficulty in tolerating doses that are of therapeutic value [3]. Secondly, older patients may take longer to respond to antidepressant medication than younger patients [2].
A related issue is the choice of which antidepressant to use. There is still no clear consensus as to first line treatment in the elderly [4] and the debate continues in the professional literature. Advocates of tricyclic antidepressants (TCAs) as first line treatment concentrate their arguments on their known efficacy in moderate to severe depression and their apparent cost-effectiveness, arguing that new products such as selective serotonin re-uptake inhibitors (SSRIs) should be reserved for those patients who cannot tolerate the side-effects of tricyclics antidepressants [5]. This argument is reinforced by the claim that the SSRIs as a class may be less effective in the treatment of severe depression, although this is based on relatively little evidence. Advocates of SSRIs as first line treatment in the elderly claim that the lower drop out rate due to adverse events represents a significant advantage for these products, and given the high morbidity and mortality associated with untreated depression, the additional benefits outweigh the additional costs [6]. This claim is also controversial since different studies have found different drop-out rates [7].
The lack of a clear consensus regarding first line treatment of the elderly has critical implications for further research in this field since a gold standard comparator cannot be clearly identified. A gold standard comparator is defined as that treatment which is the most effective and the least harmful [8]. There is currently no single agent which can be considered as ‘first-line’ treatment for elderly patients with depression. Every product has advantages and disadvantages. Flint has recently proposed that the selection of an antidepressant should be made on a case by case basis, taking account of each patient's characteristics [9].
There is a developing consensus that subjects over the age of 60 years may be considered elderly, but there should be no upper limit to the age of patients considered suitable for research. Ideally, patients aged 75 years and over should be adequately represented since this patient population differs significantly as regards key factors known to be associated with depressive morbidity, e.g. neurophysiological changes and concomitant physical illness. However, few studies have reported data on patients aged over the age of 75 years. Salzman claimed that only 240 patients aged over 75 years had been included in studies of depression, only five of whom were treated with SSRIs [10].
Whilst there are over two dozen antidepressants currently marketed in the UK [11], most studies of these drugs have been on younger adults and have excluded very old and frail patients. Exclusion criteria and recruitment sources often result in a selective trial population unrepresentative of the target population. Only a small minority of studies have included patients from the community. In addition, the few trials conducted in older adults have tended to be small and very few trials of the newer antidepressants have a placebo arm. Whilst no one class of agent has been found to be more effective than another in the acute treatment of depression in the elderly, design short-comings in many studies mean that the possibility of a real difference in efficacy between drugs cannot be excluded. The objectives of this paper are firstly to review the antidepressants currently marketed, to identify key deficiencies in the evidence supporting both older and newer products and secondly, to discuss study design challenges with respect to new products currently seeking the indication of treatment of depressive illness in the elderly.
The regulatory background
European guidelines provide advice on the study design required to support the indication ‘treatment of depressive illness’, although the elderly are not specifically addressed as a subgroup [12]. The guideline Medicinal Products for the Treatment of Depression identifies short-term efficacy in the treatment of the depressive episode and medium term efficacy in the prevention of relapse as critical to the evidence required to support the indication above. In addition, the guideline outlines the study design required to support the indication prevention of recurrence or prophylaxis of depressive illness. However, this guideline requires updating to reflect the concerns discussed above. In particular, there is a growing consensus amongst regulatory authorities in Europe for placebo-controlled trials in the elderly to both confirm efficacy and reduce the risk of obtaining a false negative result.
ICH (International Council for Harmonization) guidelines also provide advice on the evidence required to support both claims for efficacy and safety, for both short-term and long-term treatment. An ICH guideline is currently in development, which provides advice on choice of control group in clinical trials.
Currently marketed antidepressants in the UK
The British National Formulary presents information on antidepressants in the following four groups: tricyclic and related antidepressant drugs; monoamine oxidase inhibitors (MAOIs); SSRIs; and finally, a heterogenous group of other antidepressant drugs (Table 1). The advice on posology in the elderly is derived from the Summary of Product Characteristics of these products. The quality of the advice provided is variable; titration period and duration of treatment is rarely mentioned.
Table 1.
Antidepressants currently marketed in the U.K: Advice re posology in the elderly.
| Posology: Elderly | |
|---|---|
| Tricyclic and related antidepressant drugs | |
| Amitriptyline hydrochloride | Lower starting and maintenance dose |
| Amoxapine | Lower starting and maintenance dose |
| Clomipramine hydrochloride | Lower maintenance dose |
| Dothiepin hydrochloride | Lower starting and maintenance dose |
| Doxepin | Lower starting and maintenance dose |
| Imipramine | Lower starting and maintenance dose |
| Lofepramine | Yes (nonspecific) |
| Nortriptyline | Yes (nonspecific) |
| Protriptyline hydrochloride | Lower starting and maintenance dose |
| Trimipramine | Lower starting and maintenance dose |
| Maprotiline hydrochloride | Lower starting and maintenance dose |
| Mianserin hydrochloride | Lower starting dose |
| Trazodone hydrochloride | Lower starting dose |
| Viloxazine hydrochloride | Lower starting and maintenance dose |
| Monoamine oxidase inhibitors (MAOIs) | |
| Phenelzine | No specific advice |
| Isocarboxazid | Lower maintenance dose |
| Tranylcypromine | Use with great caution |
| Moclobemide | No specific advice |
| SSRIs | |
| Citalopram | Lower maximum dose |
| Fluoxetine | No specific advice |
| Fluvoxamine maleate | No specific advice |
| Paroxetine | Lower maximum dose |
| Sertraline | No specific advice |
| Other antidepressant drugs | |
| Flupenthixol | Lower starting and maintenance dose |
| Mirtazapine | No specific advice |
| Nefazodone hydrochloride | Lower maintenance dose; Slower dose titration. |
| Reboxetine | Not currently recommended in elderly |
| Tryptophan | Yes (nonspecific) |
| Venlafaxine | No specific advice |
Antidepressants can be classified according to structure or function, and may be further subdivided. Thus tricyclics (TCAs) can be subdivided into secondary amine (nortriptyline) and tertiary amine (imipramine) TCAs, tetracyclics (mianserin), and triazolopyridines (trazodone). Monoamine oxidase inhibitors (MAOIs, e.g. phenelzine), constitute another group, which can be subdivided into reversible and irreversible or selective and nonselective MAOIs, e.g. reversible inhibitor of MAO-A, or RIMA (moclobemide). Selective serotonin re-uptake inhibitors (SSRIs) are a third group, whilst noradrenaline re-uptake inhibitors form a fourth group, which can be subdivided into selective (reboxetine) and nonselective (venlafaxine) NRIs.
Finally, antidepressants such as nefazodone inhibit reuptake of serotonin and also selectively block serotonin receptors, whilst mirtazapine, a presynaptic α2-adrenoceptor antagonist, increases central noradrenergic and serotonergic neurotransmission. There are also several other antidepressants classified as ‘atypical’ not currently marketed in the U.K
First generation antidepressants
There are a large amount of data available on the efficacy and adverse effects of the first-generation antidepressants, the TCAs and nonselective MAOIs [13]. However, the number of randomised controlled trials performed in the elderly is relatively small [14]. Nortriptyline is the most extensively studied TCA in the elderly, with data from over 20 clinical trials [15]. There is also a modest amount of clinical trial evidence supporting the efficacy of the MAOI phenelzine [16].
Second generation antidepressants
The second generation antidepressants include the SSRIs, SNRIs and RIMAs. Some of the newer antidepressants have received little or no formal evaluation in elderly patients. There has been only one published double-blind controlled trial of venlafaxine in the elderly [17] and there have been no controlled studies of nefazodone in geriatric depression. Pooled analysis has been used to provide supporting evidence of efficacy for these newer products. An analysis of data from 2897 patients enrolled in phase II and III studies found that venlafaxine was comparably efficacious in young and in a subset of 357 elderly patients with depression [18]. Four double-blind, placebo controlled studies of nefazodone included patients aged up to 81 years with severe depression and melancholia, three of which used imipramine as a comparator [13]. A pooled analysis of all 247 patients found nefazodone to be effective in patients with moderate or severe depressive symptoms although no separate age analyses were presented [19].
The efficacy of moclobemide has been examined through both placebo controlled and active comparator studies. One study used a 7-week, double-blind design of moclobemide 400 mg day−1 vs nortriptyline 75 mg day−1 in 109 patients aged over 60 years with major depression. The rates of remission were not statistically significant [20]. However this study by Nair and similar studies appear to have been underpowered. In a large meta-analysis, moclobemide was found to have equal efficacy in elderly and younger patients [21].
Controlled efficacy studies
Controlled studies are considered to provide the primary source of evidence of efficacy, whilst meta-analyses can provide supporting evidence. A number of articles have reviewed the findings of controlled antidepressant trials conducted in patients aged 55 years or older [22–26]. Approximately 50 studies were identified which compared an antidepressant drug with placebo or, more commonly, with another known antidepressant agent. The established consensus of these reviews has been that no one class of antidepressant medication is more efficacious than another in the acute treatment of depressive illness in the elderly. However most of these trials contained too few patients to determine differences in efficacy.
Placebo controlled studies
Recently the Cochrane Centre conducted a methodological review of placebo controlled trials of antidepressants in the elderly (patients aged 55 years or over) to determine the efficacy of antidepressant medication compared with placebo [27]. Twenty-one placebo-controlled trials which met the review inclusion criteria were identified and the quality of included studies was reviewed against several criteria. These trials involved a total of 1942 subjects. Four hundred and ninety subjects failed to complete, resulting in approximately 1500 evaluable subjects.
The preliminary analysis suggested that some drugs do not significantly differ from placebo in treating depression in this age group. In particular, the studies using imipramine tended to show no efficacy against placebo, but this may reflect the doses used. In addition, the sample sizes were small and further studies should be undertaken. Another significant finding from these trials was the large placebo effect. The authors concluded that there is both anecdotal and research evidence to support the case that antidepressants should be the subject of specific trials in the elderly.
Active comparator controlled studies
Most of the evidence for efficacy of the newer antidepressants derives from active comparator controlled studies [9]. However, the routine use of tertiary amine TCAs, such as imipramine, as comparator drugs is an area of concern particularly since very few trials of the newer antidepressants have a placebo arm and therefore no firm evidence of efficacy per se. Whilst the efficacy of imipramine has previously been established in relation to placebo, secondary amine TCAs, such as nortriptyline are the preferred choice of TCA in the elderly, since they are less likely to induce adverse effects. A further criticism is that trials of efficacy using imipramine as an active comparator have tended to use relatively low, less efficacious doses of impramine, therefore biasing the assessment of efficacy in favour of the investigational drug.
Dose finding studies
As adults age, pharmacokinetic changes result from changes in body composition and the function of drug-eliminating organs [28], The reduction in lean body mass, serum albumin and total body water, and the increase in percentage of body fat result in changes in distribution of drugs depending on their lipid solubility and protein binding. The clearance of many drugs is reduced in the elderly. Renal function declines at a variable rate to about 50% that in the young adult. Hepatic blood flow and the function of some of the drug metabolising enzymes, e.g. the cytochrome P450 enzymes is also reduced, but conjugation mechanisms are relatively well maintained. The elimination half-life is often increased as a consequence of changes in distribution, and/or a reduction of the renal or metabolic clearance. Changes in pharmacodynamics are another important factor in the development of therapeutic agents. Drugs that depress the central nervous system produce increased effects at any given plasma concentration. Physiological changes can result in increased sensitivity to the effects of other drugs.
Age-related pharmacokinetic and pharmacodynamic changes require trials to be appropriately designed to determine the optimum starting dose, rate of titration, recommended maintenance dose and maximum dose. Four to six weeks of treatment may be necessary before dose adjustment should be considered because elderly patients may take longer to respond. A trial design that does not address these issues may result in an inaccurate benefit:risk assessment of the product.
Several pharmacokinetic studies have been performed in the elderly although results are sometimes conflicting. One study found that ageing had no effect on the plasma concentration of mianserin [29] whilst other studies have reported an age-associated increase in the elimination half-life and plasma concentration [30]. In a review of the pharmacokinetics of the SSRIs, De Vane recommended that the starting and maximum doses of citalopram and paroxetine should be reduced in the elderly [31]. However, there is controversy about the optimum dose of SSRIs [32]. Moclobemide is the most extensively studied RIMA and age has no clinically significant effect on its pharmacokinetics and thus age-related dose adjustments are not required [33]. Ageing does affect the pharmacokinetics of nefazodone, resulting in a lower starting dose, slower dose titration and a lower therapeutic dose range. The newer antidepressants also recommend dosage reduction in patients with renal impairment (venlafaxine) or hepatic cirrhosis (venlafaxine, nefazadone), reflecting the results of pharmacokinetic studies [13].
However, very few formal dose finding studies have been performed in the elderly to identify optimum dose regimens. Non-selective MAOIs appear to have a linear dose–response but the optimal dose in older patients has not been established. Although age-related dose adjustments for newer MAOIs such as moclobemide are not required, the optimal dose in older patients has not been established [13]. Similarly the usual therapeutic dose of venlafaxine in older patients has not yet been established, although the dose range is wide.
Prevention of relapse/recurrence studies
Demonstration of prevention of relapse (continuation therapy) in elderly patients has rarely been addressed in controlled trials. Georgatas et al. reported rates of relapse of 16.7% for nortriptyline and 20% for phenelzine in elderly depressed patients during 4–8 months of continuation therapy [34]. Reynolds also found that nortriptyline was effective in preventing further recurrence in elderly patients who had recovered from a recurrent episode of major depression [35]. After 1 year of treatment, 80% of patients administered nortriptyline remained free of major depression, compared with only 20% of patients taking placebo. Despite this study and a 2 year study of dothiepin [36], evidence for prevention of recurrence of depression is rare and mainly limited to comparisons of TCAs and lithium. To date, there are no published controlled studies on the use of SSRIs or other new antidepressants in the long term treatment of depression in the elderly. However, such trials are currently being conducted [37].
Meta-analyses
Meta-analysis may provide evidence of efficacy, where single under-powered studies have failed, as discussed above. Formal meta-analyses should combine trials with similar designs, treatment groups and endpoints to provide supporting evidence of efficacy or to differentiate between treatments. The meta-analyses performed often do not meet all of these criteria. A recent meta-analysis which compared TCAs, SSRIs, reversible inhibitors of MAO-A (RIMAs) and ‘atypical’ antidepressants in geriatric depression found that patients treated with SSRIs had a higher response rate than those given atypical antidepressants [7]. The generalization of the latter finding is debatable, since it was based on only two studies (fluoxetine vs trazodone and paroxetine vs mianserin).
Safety studies
Controlled trials can provide evidence of comparative safety of new agents. Specific safety studies are however, also indicated. Interaction studies are of particular relevance since the use of concomitant medication is very common in the elderly population. Ideally, interaction studies should be performed with the most commonly used concomitant psychotropic medications and on the basis of the known pharmacology of the agent. Information on absorption, distribution, metabolism and excretion should be obtained for both the active drug and the main metabolites, since the latter may be more pharmacologically active or have a longer half-life. The SSRI, fluoxetine, has an elimination half-life of 70 h and its active metabolite, norfluoxetine, has a half-life of 330 h. Whilst there is considerable information relating to the pharmacology of the SSRIs, these drugs are a structurally heterogeneous group and this is reflected in their pharmacokinetics [38, 39].
It is now well established that the SSRIs inhibit CYP isoenzymes [40], although there are differences in their potency of inhibition [41]. The clinical significance of many potential SSRI drug interactions is not known, although there appears to be a correlation between the adverse effect profile and enzyme inhibition. Paroxetine has a less favourable adverse effect profile than sertraline or citalopram, as reflected in its potency of CYP isoenzyme inhibition. In contrast to the SSRIs, there has been very little evaluation of the effect of TCAs on the hepatic cytochrome P450 (CYP) system. Nortriptyline and desipramine are metabolized by CYP2D6 [41], which is the major isoenzyme involved in the metabolism of CNS agents. Most of the TCAs are metabolized in the liver and undergo demethylation and hydroxylation. However, the known pharmacological characteristics of some of the newer agents are incomplete, for example knowledge of the main metabolites.
Therefore the evidence for efficacy and safety of antidepressants in the elderly is far from convincing, relying often on supporting data from meta-analyses rather than on pivotal, adequately designed controlled trials. Dose finding studies in the elderly and clinical interaction studies are rarely performed although in vitro pharmacokinetic data of limited clinical utility is often provided. Population pharmacokinetic data from Phase III clinical trials or postmarketing studies are occasionally available and may be useful in defining the optimum dose in the elderly and other subpopulations (G. Tucker, personal communication).
Challenges for study design and interpretation
Drug development undergoes several phases:
Phase I: Small pharmacokinetic studies in healthy volunteers, including healthy elderly people.
Phase II: Pilot efficacy, safety and dose finding studies in small numbers of patients.
Phase III: Clinical efficacy and safety studies in larger patient populations.
Phase IV: Post-marketing safety studies, to identify unusual or infrequent ADRs or drug interactions. Can also provide population pharmacokinetic data.
A bridging study (a PhaseI/II study in patients) is sometimes used.
The design of studies of antidepressant efficacy and safety in the elderly needs to address all the issues discussed above, in addition to addressing the requirements of the current guidelines.
Clinical trial programme
A proposed Phase II and III clinical trial programme which should provide adequate evidence of efficacy and safety in support of new antidepressant agents, i.e. prior to obtaining a Product Licence or Marketing Authorization is outlined below.
Critical Phase II dose finding studies need to be performed in elderly patients, where patients aged above 75 years and those with concomitant illness are also represented. These studies should be conducted in small numbers of patients, often in an in-patient setting, where patients can be closely monitored, and therefore exclusion criteria should be kept to the minimum. A double-blind, randomised trial using a fixed dose panel has advantages in this group of patients. In such a trial, several patients are randomly allocated to a prespecified dose (which may require titration), where they should be stabilized for a duration of 8 weeks to ensure optimal treatment response. A placebo group should also be included to provide preliminary evidence of efficacy, especially where a dose response relationship does not exist. This study should identify an optimum dose based on a benefit:risk assessment of efficacy and safety, or an upper and lower therapeutic dose.
Figure 1 presents a three step Phase III clinical trial programme, with two different doses of the investigational drug (ID) previously identified in Phase II studies. The first step represents a four arm acute efficacy study, with patients randomised to placebo, active comparator or investigational drug. Ideally, two different doses of the investigational drug should be included, in order to identify the optimum dose, unless this has been established in Phase II dose-finding studies. In step 2 responders to the investigational drug at 8 weeks are re-randomised to either placebo or the maintenance dose of the investigational drug, and are then followed up for a further 3–6 months to identify differences in relapse rates between treatments. The evidence of efficacy provided by such a trial would be adequate to support the indication treatment of depressive illness in the elderly, given positive evidence of superiority, and a favourable benefit:risk profile of the investigational drug. In step 3, patients who had recovered from their depressive episode could continue on either placebo or investigational drug for a further 12 months, in order to evaluate prevention of recurrence.
Figure 1.
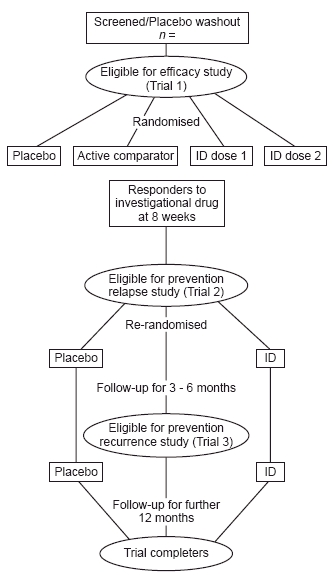
Clinical trial programme.
An alternative trial design would require responders at 6 months to enter a 4 week placebo phase in order to select patients who had recovered from their depressive episode (the criteria for recovery should be prespecified in the protocol). These patients should then be randomised to placebo or active drug for a further period of 9–12 months, or preferably longer, in order to evaluate prevention of recurrence. Positive evidence of prevention of recurrence could be used to support the indication prevention/prophylaxis of depressive illness in the elderly.
Clinical trial protocol
Key issues to be addressed in the protocol are the main objectives of the trial, the primary and secondary endpoints and whether the study has been designed as a superiority or noninferiority trial. Inclusion criteria need to specify diagnostic criteria (Table 2) for major depressive episode [43] and the minimum score required on the chosen rating scale. The exclusion criteria should be kept to the minimum, ensuring that patients with concomitant illness are represented (unless there are specific safety concerns) and there should be no upper age limit to trial recruitment. The trial should aim to recruit a significant proportion of patients aged over 75 years.
Table 2.
Simplified diagnostic criteria for major depressive episode.
| Five or more of the following symptoms have been present nearly every day for 2 weeks; at least one of the symptoms is either (a) or (b). |
| (a) Depressed mood most of the day |
| (b) Markedly diminished interest or pleasure in all or almost all activities most of the day |
| (c) Significant weight loss or weight gain or decrease or increase in appetite |
| (d) Insomnia or hypersomnia |
| (e) Psychomotor agitation or retardation |
| (f) Fatigue or loss of energy |
| (g) Feelings of worthlessness or excessive or inappropriate guilt (which may be delusional) |
| (h) Diminished ability to think or concentrate, or indecisiveness |
| (i) Recurrent thoughts of death, recurrent suicidal ideation, or suicide attempt or a specific plan for committing suicide |
| Symptoms cause clinically significant distress or impairment in important areas of functioning |
| Symptoms are not due to the direct psychological effects of a substance or a general medical condition |
| Symptoms are not better accounted for by bereavement |
Randomization techniques should be used, preferably with some form of central randomization for multicentre trials. Evidence of baseline comparability between treatment groups should be presented. The treatment protocol must be clearly specified, with starting and maximum doses and rate of titration described. The trial must be of adequate duration (see Figure 1).
Measurement methods need to be well validated and both patients and the raters need to be blinded to treatment. Quality of life questionnaires should be incorporated, e.g. the Montgomery Asberg Depression rating scale (MADRS) as well as key rating scales such as the Hamilton Depression rating scale (HAM-D). Relapse and recurrence needs to be clearly predefined in relation to a specified duration of time between resolution and re-emergence of symptoms (using an appropriate measurement scale). Criteria for response to treatment also need to be predefined in the protocol, again using an appropriate measurement scale. The study must be adequately powered to allow for differential drop-outs in the treatment groups. There are likely to be different drop-out rates in the placebo and active treatment groups, with patients in the former more likely to discontinue due to lack of efficacy and patients in the latter more likely to discontinue due to adverse events.
All analyses (including any interim analysis) should be prespecified in the protocol and correct procedures followed to prevent the introduction of bias. Any protocol amendments should ideally be made before the trial has begun. An Intent to Treat analysis should be performed in a superiority trial. Ideally both a LOCF (last observation carried forward) and an observed case analysis should be performed. A Per Protocol analysis should also be performed in a noninferiority trial. Results should be presented nonselectively with tests of significance and 95% confidence intervals to enable correct interpretation. Robustness of the results should be discussed and any discrepancies should be clearly highlighted.
Discussion and conclusions
The proportion of the population considered elderly and very elderly is increasing. These individuals are more often affected by concomitant illnesses than are younger patients and consume a disproportionate share of medication. These factors combined with the ageing associated changes in pharmacokinetics and pharmacodynamics result in a patient population vulnerable to serious adverse drug effects and drug interactions. This population should therefore only receive drugs when absolutely necessary for well-defined indications and at the lowest effective dose.
This paper has reviewed pharmacotherapeutic trials of antidepressants and identified deficiencies in the evidence supporting both the efficacy of these agents in the elderly and the optimum dosage regimen. Fundamental problems remain with extrapolating the results of studies in a highly selective trial population, without significant comorbidity and under-representation of the very elderly, to the target elderly population.
Whilst there are considerable pharmacokinetic data, formal dose finding studies are relatively rare. Placebo controls have been infrequently included in efficacy studies of the newer antidepressants and the active comparator used is often suboptimal. A placebo control can both confirm efficacy and reduce the risk of obtaining a false negative or false positive result and thus assist in the long term development of new therapeutic agents.
It is acknowledged that receiving a placebo control in a clinical trial setting may deprive a patient of therapeutic benefit. However, given the large placebo response often found in such trials and if adequate supervision of patients is provided (and suicidal patients are excluded), this disadvantage can be minimized. Therefore, whilst there are ethical arguments against the use of a placebo in the elderly, these are outweighed by ethical arguments in favour of such a control, whereby future patients should benefit from a clearer definition of the AE profile. Elderly people are generally more susceptible to anticholinergic effects, and thus most likely to gain from this information [43]. The need for a placebo control in trials of antidepressants in the elderly is therefore gaining acceptance.
Dosage is also an important therapeutic issue, which must be addressed by the clinical trial. The benefit:risk assessment must evaluate the risks associated with both suboptimum dose and excessive dose. There is a growing body of evidence that suggests that TCAs in particular are prescribed by general practitioners at subtherapeutic dosages to the elderly and are therefore potentially harmful.
Finally, non completion of treatment is particularly common in the elderly and can significantly affect trial quality and the assessment of efficacy. The reason for discontinuation is not often recorded or provided and analyses do not always allow for patients lost to follow-up. Therefore clinical trials in elderly people must make every effort to follow-up patients who have discontinued treatment, to document the reason and continue efficacy assessments to provide comprehensive data.
In conclusion, there is considerable scope for improvement in the design and conduct of clinical trial programmes in support of new antidepressant medications. The clinical pharmacology of new active substances needs to be comprehensively described and understood. Phase II studies should address optimum dosage regimens in the elderly. Specific Phase III trials are also required in the elderly to determine the efficacy and safety of antidepressants in the treatment of depressive illness. These studies need to be adequately designed and powered to take into account the high drop-out rates and the high placebo response in this age group. Given the chronic or recurrent nature of depressive illness, long-term prevention studies are desirable, although these are not currently required by European guidelines. Finally, Phase IV studies are required to provide further evidence of safety, including critical drug interaction studies, given that elderly patients often take drugs for somatic disorders.
Although this paper has not reviewed other treatment modalities, the use of cognitive and behavioural therapy as monotherapy or adjuvant treatment is increasingly recognized. These approaches may offer advantages over traditional pharmacotherapy in the elderly through a reduction in risks or side-effects associated with treatment. The future of research in this area should address both the specific issues involved in the design of pharmacotherapeutic trials and trials of adjuvant or alternative treatment modalities. Future trial designs need to be more creative, exploring combination treatment programmes, e.g. pharmacotherapy with cognitive therapy or psychotherapy to identify optimum treatment regimens [44] in this age group.
References
- 1.Saunders PA, Copeland JRM, Dewey ME, et al. The prevalence of dementia, depression and neurosis in later life. The Liverpool MRC-Alpha Study. Int J Epidemiology. 1993;22:838–847. doi: 10.1093/ije/22.5.838. [DOI] [PubMed] [Google Scholar]
- 2.Reynolds C, Frank E, Kupfer DJ, et al. Treatment outcome in recurrent major depression. Am J Psychiatry. 1996;153:1288–1292. doi: 10.1176/ajp.153.10.1288. [DOI] [PubMed] [Google Scholar]
- 3.Schneider LS, et al., editors. Diagnosis and treatment of depression in late life. Washington, DC: American Psychiatric Press; 1994. Results of the NIH Consensus Development Conference; pp. 181–244. [Google Scholar]
- 4.Baldessarini RJ. Drugs and the treatment of psychiatric disorders: Depression and mania. In: Goodman, Gilman's, editors. The Pharmacological Basis of Therapeutics. 1996. [Google Scholar]
- 5.Edwards JG. Prevention of relapse and recurrence of depression: newer versus older anti-depressants. Adv Psych Treatment. 1997;3:52–57. [Google Scholar]
- 6.Tobiansky R. Impact of side effects of treatment is important in older patients. Br Med J. 1998;317:1157. [PubMed] [Google Scholar]
- 7.Mittman N, Herrman N, Einarson TR, et al. The efficacy, safety and tolerability of antidepressants in late life depression: a meta-analysis. J Affect Disorders. 1997;46:191–217. doi: 10.1016/s0165-0327(97)00107-9. [DOI] [PubMed] [Google Scholar]
- 8.Eddy DM. Principles for making difficult decisions in difficult times. JAMA. 1994;271:1792–1798. [PubMed] [Google Scholar]
- 9.Flint AJ. Choosing appropriate anti-depressant therapy in the elderly: a risk: benefit assessment of available agents. Drugs Aging. 1998;4:269–280. doi: 10.2165/00002512-199813040-00003. [DOI] [PubMed] [Google Scholar]
- 10.Salzman C, Schneider L, Lebowitz B. Antidepressant treatment of very old patients. Am J Geriatric Psychiatry. 1993;1:21–29. doi: 10.1097/00019442-199300110-00004. [DOI] [PubMed] [Google Scholar]
- 11.British National Formulary (BNF) 1998. 36 September. BMA/RPSGB.
- 12.Medicinal products for the treatment of depression. The Rules Governing Medicinal Products in the European Union. 1998.
- 13.Goldberg RJ. Antidepressant use in the elderly. current status of nefazodone, venlafaxine and moclobemide. Drugs Aging. 1997;2:119–131. doi: 10.2165/00002512-199711020-00004. [DOI] [PubMed] [Google Scholar]
- 14.Salzman C. Pharmacological treatment of depression in elderly patients. In: Schneider LS, et al., editors. Diagnosis and Treatment of Depression in Late Life. Washington, DC: American Psychiatric Press; 1994. pp. 181–244. [Google Scholar]
- 15.Katz IR, Simpson GM, Curlick SM, et al. Pharmacologic treatment of major depression for elderly patients in residential care settings. J Clin Psychiatry. 1990;51(Suppl):41–47. [PubMed] [Google Scholar]
- 16.Georgatas A, McCue RE, Hapworth W, et al. Comparative efficacy and safety of MAOI vs TCAs in treating depression in the elderly. Biol Psychiatry. 1986;21:1155–1156. doi: 10.1016/0006-3223(86)90222-2. [DOI] [PubMed] [Google Scholar]
- 17.Mahapatra SN, Hackett D. A randomised, double-blind, parallel-group comparison of venlafaxine and dothiepin in geriatric patients with major depression. Int J Clin Pract. 1997;51:209–213. [PubMed] [Google Scholar]
- 18.Montgomery S. Venlafaxine: a new dimension in antidepressant pharmacotherapy. J Clin Psychiatry. 1993;54:119–126. [PubMed] [Google Scholar]
- 19.Marcus R, Mendels J. Nefazodone in the treatment of severe, melancholic, and recurrent depression. J Clin Psychiatry. 1996;57(Suppl 2):19–23. [PubMed] [Google Scholar]
- 20.Nair NP, Ahmed SK, Kin NM, et al. Reversible and selective inhibitors of monamine oxidase A in the treatment of depressed elderly patients. Acta Psychiat Scand Suppl. 1995;386:28–35. doi: 10.1111/j.1600-0447.1995.tb05921.x. [DOI] [PubMed] [Google Scholar]
- 21.Fitton A, Faulds D, Goa KL. Moclobemide: a review of its pharmacological properties and therapeutic use in depressive illness. Drugs. 1992;43:561–596. doi: 10.2165/00003495-199243040-00009. [DOI] [PubMed] [Google Scholar]
- 22.Gerson SC, Plotkin DA, Jarvik LF. Antidepressant drug studies. 1964–86: empirical evidence for aging patients. J Clin Psychopharmacol. 1988;8:311–322. [PubMed] [Google Scholar]
- 23.Volz HP, Moller HJ. Antidepressant drug therapy in the elderly: a critical review of the controlled clinical trials conducted since 1980. Pharmacopsychiatry. 1994;27:93–100. doi: 10.1055/s-2007-1014286. [DOI] [PubMed] [Google Scholar]
- 24.Anstey K, Brodarty H. Antidepressants and the elderly: double-blind trials 1987–92. Int J Geriatr Psychiatry. 1995;10:265–279. [Google Scholar]
- 25.Menting JEA, Honig A, Verhey FRJ. Selective serotonin reuptake inhibitors in the treatment of elderly depressed patients: a qualitative analysis of the literature on their efficacy and side-effects. Int Clin Psychopharmacol. 1996;11:165–175. doi: 10.1097/00004850-199609000-00003. [DOI] [PubMed] [Google Scholar]
- 26.Schneider LS. Pharmacologic considerations in the treatment of late-life depression. Am J Geriatr Psychiatry. 1996;4(Suppl 1):S51–S65. [Google Scholar]
- 27.Wilson K, Mottram P, Sivananthan A, Whalley A. A review of placebo-controlled antidepressant drug trials in the treatment of older depressed people. University of Liverpool/Cochrane Collaboration Depression Anxiety Neurosis Cochrane Library, 1999; in press.
- 28.Goodman, Gilman's . In: The Pharmacological Basis of Therapeutics. Gilman AG, Hardman JG, Limbird LE, editors. McGraw Hill; 1996. pp. 431–460. [Google Scholar]
- 29.Leionen E, Koponen H, Lepola U. Serum mianserin and ageing. Prog Neuropsychopharmacol, Biol Psychiatry. 1994;18:833–845. doi: 10.1016/0278-5846(94)90100-7. [DOI] [PubMed] [Google Scholar]
- 30.Begg EG, Sharman JR, Kidd JE, Sainsbury R, Clark DWJ. Variability in the elimination of mianserin in elderly patients. Br J Clin Pharmacol. 1198;27:445–451. doi: 10.1111/j.1365-2125.1989.tb05392.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.De Vane CL. Pharmacokinetics of the selective serotonin reuptake inhibitors. J Clin Psychiatry. 1992;53(Suppl 2):13–20. [PubMed] [Google Scholar]
- 32.Rifkin A. SSRI optimal dose remains at issue. J Clin Psychiatry. 1997;58:87–88. doi: 10.4088/jcp.v58n0206d. [DOI] [PubMed] [Google Scholar]
- 33.Stoeckel K, Pfefen JP, Mayersohn M, et al. Absorption and disposition of moclobemide in patients with advanced age or reduced liver and kidney function. Acta Psychiatr Scand Suppl. 1990;360:94–97. doi: 10.1111/j.1600-0447.1990.tb05346.x. [DOI] [PubMed] [Google Scholar]
- 34.Georgatas A, McCue RE, Cooper TB, et al. A placebo-controlled comparison of notriptyline and phenelzine in maintenance therapy of depressed elderly patients. Arch Gen Psychiatry. 1989;46:783–786. doi: 10.1001/archpsyc.1989.01810090025004. [DOI] [PubMed] [Google Scholar]
- 35.Reynolds CF, Frank E, Perel JM, et al. Maintenance therapies for late-life recurrent major depression: research and review circa 1995. Int Psychogeriatr. 1995;7(Suppl):27–39. doi: 10.1017/s104161029500233x. [DOI] [PubMed] [Google Scholar]
- 36.Old Age Depression Interest Group. How long should the elderly take anti-depressants? A double-blind placebo-controlled study of continuation/prophylaxis therapy with dothiepin. Br J Psychiatry. 1993;162:175–182. [PubMed] [Google Scholar]
- 37.Wilson K, Mottram P, Ashworth L. Continuation and maintenance studies with antidepressants in older people; a critical review and preliminary data. J Serotonin Res. 1997;4(suppl.):1–16. [Google Scholar]
- 38.Grimsley SR, Jann MW. Paroxetine, setraline and fluvoxamine: new selective serotonin reuptake inhibitors. Clin Pharm. 1992;11:930–957. [PubMed] [Google Scholar]
- 39.Rickels K, Schweizer E. Clinical overview of serotonin reuptake inhibitors. J Clin Psychiatry. 1990;51(12 Suppl B):9–12. [PubMed] [Google Scholar]
- 40.Nemeroff CB, DeVane CL, Pollock BG. Newer antidepressants and the cytochrome P450 system. Am J Psychiatry. 1996;153:311–320. doi: 10.1176/ajp.153.3.311. [DOI] [PubMed] [Google Scholar]
- 41.Crewe HK, Lennard MS, Tucker GT, Woods FR, Haddock RE. The effect of selective serotonin re-uptake inhibitors on cytochrome P450 2D6 activity in human liver microsomes. Br J Clin Pharmacol. 1992;34:262–265. doi: 10.1111/j.1365-2125.1992.tb04134.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4. Washington, DC: APA; 1994. [Google Scholar]
- 43.Spigset O, Mantensson B. Fortnighly review: Drug treatment of depression. Br Med J. 1999;318:1188–1191. doi: 10.1136/bmj.318.7192.1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Malt UF, Robak OH, Madsbu HP, Bakke O, Loeb M. The Norwegian naturalistic treatment study of depression in general practice (NORDEP) -I: randomised double blind study. Br Med J. 1999;318:1180–1184. doi: 10.1136/bmj.318.7192.1180. [DOI] [PMC free article] [PubMed] [Google Scholar]


