Abstract
Aims
In October 1995 in response to the results of three studies, the Committee on the Safety of Medicines advised doctors and pharmacists that oral contraceptives containing desogestrel (DSG) and gestodene (GST) were associated with around a two-fold increase in the risk of thromboembolism compared with those containing other progestogens. The objective of this study was to estimate the risk of idiopathic venous thromboembolic disease (VTE) in users of combined oral contraceptives (COCs), to compare the risk between formulations and to examine the effect of using age banding as opposed to matching by exact year of birth.
Methods
A nested case control study was conducted using the General Practice Research Database. Women with a VTE event recorded between 1992 and 1997, who were treated with an anticoagulant, from consideration of their prescription records were likely to have been using a COC prescription on the day of the event and also had no exclusion factors, were deemed cases. For comparison with the previous studies, two nested case control studies were undertaken. Study 1 used controls matched by practice and year of birth. Study 2 used controls matched by practice and within 5 years age bands.
Results
We found an incidence of idiopathic VTE amongst users of combined oral contraceptives of 3.8 per 10 000 exposed women years. Incidence rates increased markedly after 35 years of age. The nested case-control study using controls matched by year of birth showed no significant difference in risk between the major COC formulations. With levonorgestrel (LNG) 150 µg and ethinyloestradiol (EE) 30 µg as the reference, the adjusted ORs for GST 75 µg and EE 30 µg was 1.3 (95% CI 0.8, 2.1), for DSG 150 µg and EE 30 µg it was 1.0 (95% CI 0.7, 1.7) and for DSG 150 µg and EE 20 µg it was 0.8 (95% CI 0.4, 1.6). Using less rigorous matching criteria, matching controls to cases within 5 years age bands, the ORs increased. When a mixed group of COCs, characterized by having LNG as the progestogen component was used as the reference category, there was an elevation in the ORs for the newer products. We found a significant association between idiopathic VTE and current smoking (OR 2.0 (1.4, 2.7)), BMI over 35 (OR 3.8 (1.8, 8.0)) and asthma (OR 1.9 (1.3, 2.9)). The OR for women who had proxy evidence of general ill health (indicated by the number of prescriptions issued) was 2.2 (1.7, 3.7).
Conclusions
The results of this study indicate that a number of the characteristics of the women taking COCs affect the risk of VTE. There is no evidence to support the hypothesis that there is any difference in risk between COC formulations containing under 50 µg ethinyloestradiol.
Keywords: database studies, oral contraceptives, pill scare, risk factors, venous thromboembolism
Introduction
In October 1995 the UK Committee on Safety of Medicines (CSM) informed doctors and pharmacists that combined oral contraceptives (COCs) containing desogestrel (DSG) or gestodene (GST) were associated with a two-fold increase in risk of venous thromboembolic disease (VTE) [1]. They advised that these products should only be used by women who were intolerant of other combined oral contraceptives and were prepared to accept an increased risk of venous thromboembolism. On publication of the pivotal studies [2–4] it was apparent that the differences in the risk of VTE between COCs containing different progestogens were not as clear as were implied in the CSM’s letter [5]. The reported odds ratios (ORs) for DSG compared with LNG varied between 1.5 and 4.8 and between 0.9 and 5.3 for GST. The highest odds ratios were found in the non-UK centres of the WHO study (Table 1).
Table 1.
Findings from the major studies
| Study | Product | Exposed cases | OR | 95% confidence interval | |
|---|---|---|---|---|---|
| WHO Non-Oxford Centres | LNG | 97 | Reference group | ||
| DSG | 7 | 4.8 | 0.5 | 43.4 | |
| GST | 16 | 5.3 | 1.8 | 15.5 | |
| WHO Oxford Hospital Controls | LNG | 40 | Reference group | ||
| DSG | 28 | 2.3 | 1.1 | 4.9 | |
| GST | 20 | 2 | 0.8 | 4.7 | |
| WHO Oxford Community Controls | LNG | 36 | Reference group | ||
| DSG | 27 | 1.8 | 0.7 | 4.8 | |
| GST | 16 | 0.9 | 0.3 | 2.8 | |
| Transnational Germany | 2nd generation | 68 | Reference group | ||
| DSG | 12 | 1.5 | 0.8 | 3.1 | |
| GST | 10 | 2.6 | 1 | 7.2 | |
| Transnational UK | 2nd generation | 64 | Reference group | ||
| DSG | 53 | 1.6 | 1 | 2.5 | |
| GST | 45 | 1.4 | 0.9 | 2.3 | |
| BCDSP – GPRD | LNG | 23 | Reference group | ||
| DSG | 30 | 2.2 | 1.1 | 4.4 | |
| GST | 23 | 2.1 | 1 | 4.4 | |
| UK – MediPlus (1997) | LNG | 24 | Reference group | ||
| DSG | 32 | 0.9 | 0.4 | 1.8 | |
| GST | 22 | 0.8 | 0.4 | 1.9 | |
Note: Statistically significant ORs (at the 5% level) are marked in bold.
For many years it has been accepted that there is an oestrogen dose response relationship in the risk of VTE [6–8]. The hypothesis that risk varies according to the progestogen component is new and has attracted wide attention [9–11]. The studies that prompted the CSM’s decision focused on groups of products rather than specific formulations. Over 30 formulations of COC containing different types and dosages of oestrogen and progestogen are available in the UK. The characteristics of women using specific formulations differ. Many products are used by less than 2% of the population and it is thus understandable that the investigators chose to aggregate products. Although aggregation increases the statistical power, it may distort the assessment of the risks associated with any or all of the individual products. In the UK between 1992 and 1995 about 75% of all COC prescriptions were for one of four formulations: LNG 150 µg + ethinyloestradiol (EE) 30 µg (Microgynon/Ovranette)[24%], DSG 150 µg + EE 30 µg (Marvelon)[20%], GST 75 µg + EE 30 µg (Femodene/Minulet)[18%] and triphasic LNG + EE (Logynon/Trinordiol)[13%]. This usage pattern allows for the risks of VTE associated with the main formulations to be compared. In 1997 we published the results of our investigation using the UK MediPlus database [12]. In that study we used controls matched to the cases by practice and year of birth. In this respect the MediPlus study differed from the WHO and Transnational studies that used hospital controls and matched within 5 years age bands. The ORs found in the MediPlus study were lower than those found in the previous studies. This was attributed in part to the exact age matching.
The objectives of this study were to compare the risks of idiopathic VTE amongst users of different combined oral contraceptives and to explore the effects of precise age matching of controls. The study covers events that were recorded in the UK General Practice Research Database (GPRD) between 1992 and 1997. There was a substantial change in patterns of OC utilization following the advice of the CSM in October 1995. We chose to include post 1995 data in this study in order to explore the hypothesis that COCs containing GST or DSG carry an increased risk of VTE due to their specific pharmacological action. Were the hypothesis to be true then there would be no change in adjusted odds ratios following the change in patterns of utilization.
Methods
The GPRD database has been described elsewhere [13, 14] and has been used extensively in epidemiological research for studies concerned with the assessment of the safety of medicines. We used data from all the 618 practices that were deemed to have been producing data of research quality by the management committee of the GPRD.
This study was limited to events that occurred between January 1992 and June 1997. We identified all prescriptions for combined oral contraceptives issued to women between 1991 and 1997 who were aged between 15 and 49 years at the time the prescription was issued. The 1991 prescriptions were identified in order to include contraception that was prescribed before yet used during the study period. For each patient each prescription was mapped to the day on which oral contraception was being used. Where the period covered by a prescription was overlapped by a prescription for another product then the exposure to the first product was truncated at the time of the change in product and the woman was deemed to have been exposed to the second product thereafter. Using this method the oral contraceptives that would not have been used were removed from the denominator. For each calendar year and for each product and for each year of age the total number of days of exposure (including the pill free period) were summed to provide the denominator for the cohort rate estimates. The mapped exposure matrix for each individual woman on the database was used to identify women who were exposed to an oral contraceptive on specific days and to calculate the numbers of women years of exposure during each calendar year.
The data base was searched for all women who had a diagnosis of deep venous thrombosis, pulmonary embolism or venous thromboembolism not otherwise specified, a record of a prescription of a COC and evidence of treatment with an anticoagulant. Admission to hospital was not adopted as one of the search criteria because a coded record of the admission is not always entered on the database because the patient may be admitted without the general practitioner being aware. The full computer record of each of the women was printed and assessed independently by three teams. Women were accepted as cases of VTE if they had one of the above diagnoses followed by evidence of treatment with an anticoagulant, were exposed to a COC and were aged between 15 and 49 years on the day of the event.
In order to focus on idiopathic VTE women were excluded as cases if:
They were pregnant at the time of the event (delivery up to 38 weeks after the event date)
-
Within 42 days prior to the event they:
Had delivered (postpartum)
Had a termination of pregnancy
Had surgery requiring a general anaesthetic
Had major trauma to the lower limbs
Had evidence of malignant disease
Were using other sex hormones concurrently with the oral contraceptive
Had significant congenital heart disease
The event was associated with a drug overdose.
Women were also excluded if there was less than 6 months of research standard data available in the record prior to the event. (The management committee of the GPRD determines when the data supplied by each practice is of research standard based on the completeness and accuracy of records.) All final decisions regarding inclusion or exclusion were made without knowledge of the COC brand names or formulations to which the women were exposed.
The database was also searched for women who died from pulmonary embolism, deep venous thrombosis or related cause, and who had a record of hormonal contraception up to 140 days prior to the date of death. A further search identified women whose cause of death was not recorded and who could have been using an OC at the time of death. An anonymised copy of the death certificate was sought for all these deaths. Those certified as due to VTE were accepted as cases. The inclusion criteria for fatal cases were the same as nonfatal cases except that evidence of anticoagulant treatment was not a requirement. Nine women met the criteria, all of whom died from pulmonary embolism.
For those patients still registered with their practice when this investigation was undertaken, letters were sent to the doctor requesting additional information regarding the diagnosis and treatment of the woman including ascertainment of whether or not she had been admitted to hospital. Enquiries were not pursued where patients had transferred to another practice on the assumption that additional records would have been sent on to the new practice.
We examined the overall patterns of utilization of COCs and computed the cohort incidence rates of VTE. We then undertook two nested case-control studies. Where possible, four controls were randomly matched to each case. The controls were subject to the same exclusion criteria as the cases. The first study used controls matched by practice and year of birth and who were exposed to a combined oral contraceptive on the day of the event of the case. The second group of controls were matched by practice and within 5 years age bands (i.e. a woman aged between 15 and 19 years was matched to other women aged 15–19 years) and were also exposed to a combined oral contraceptive on the day of the event of the case. There were no shared controls. From the records of both cases and controls we abstracted the BMI, blood pressure and smoking status records closest to the event date. Duration of exposure to any COC was recorded for each case and control. The number of nonoral contraceptive prescriptions issued in the 6 months prior to the event date was abstracted as a general health indicator. The presence of any chronic disease such as asthma or thyroid disease was also recorded.
The primary analyses were conditional logistic regressions (computed using STATA) focusing on formulation rather than progestogen.
Results
Utilization
Material was available from 618 practices that had been providing data of research standard to the GPRD at some time between 1992 and 1997. The database included the records of about 1.1 million women who were aged between 15 and 49 years at any time during the study period. During this period there was a total of 5.3 million women years of observation. There were about 800 thousand women years exposed to oral contraceptives. The most frequently prescribed oral contraceptives are shown in Table 2 together with the mean ages of the users between 1992 and 1997 and the proportion for each product used by women under 25 years and those above 35 years. Four products account for 75% of total use. The age distribution of the users of all products is skewed. With the exception of users of DSG 150 µg + EE 20 µg, the users of the LNG products were older than the users of DSG, GST and norgestimate (NRG) products. DSG 150 µg + EE 20 µg has the highest proportion of users over the age of 35 years (27.3%). Both of the main sequential products were used by women older than the users of the monophasic preparations. Users of LNG 250 µg + EE 30 µg were older than users of LNG 150 µg + EE 30 µg by an average of nearly 3 years.
Table 2.
Exposed Woman Years (15–49-year-olds) to COCs for 1992–97.
| Formulation | EWY⋆ | Mean age (years) | Cases | Rate per 10000 EWY⋆ | Rate ratio (95% CI) | Percentage of total use | used by women under 25 years | used by women over 35 years |
|---|---|---|---|---|---|---|---|---|
| LNG 150 µg + EE 30 µg | 190191 | 27.4 | 64 | 3.4 | REFERENCE | 24.3 | 34.5 | 13.1 |
| DSG 150 µg + EE 30 µg | 152524 | 25.8 | 65 | 4.3 | 1.3 (0.9,1.8) | 19.5 | 46.6 | 8.7 |
| GST 75 µg + EE 30 µg | 143581 | 25.9 | 63 | 4.4 | 1.3 (0.9,1.9) | 18.3 | 46.6 | 9.6 |
| Triphasic LNG (50 75 125) + EE (30 40 30) µg | 102787 | 28 | 26 | 2.5 | 0.8 (0.5,1.2) | 13.1 | 27.4 | 12.6 |
| DSG 150 µg + EE20 µg | 37584 | 28.8 | 18 | 4.8 | 1.4 (0.8,2.4) | 4.8 | 37.7 | 27.3 |
| NRG 250 µg + EE 35 µg | 40440 | 25.8 | 15 | 3.7 | 1.1 (0.6,2.0) | 5.2 | 47.6 | 9.7 |
| NRT 500 µg + EE 35 µg | 22981 | 27.5 | 5 | 2.2 | 0.6 (0.2,1.6) | 2.9 | 33.3 | 12.4 |
| Cyproterone acetate 2 mg + EE 35 µg | 25709 | 25.6 | 16 | 6.2 | 1.8 (0.9,3.2) | 3.3 | 47.6 | 8.7 |
| Triphasic NRT + EE 35 µg | 16440 | 27.8 | 7 | 4.4 | 1.3 (0.5,2.8) | 2.1 | 27.8 | 11.4 |
| LNG 250 µg + EE 30 µg | 14092 | 30.1 | 8 | 5.7 | 1.7 (0.7,3.5) | 1.8 | 22.4 | 23.6 |
Total of 783 876 exposed women years to COCs. Other formulations account for 37547 EWY.
There were substantial changes in the overall pattern of use of COCs between 1992 and 1997 (Figure 1). From 1992 to 1994 GST, DSG and, latterly NRG based products displaced LNG products. After 1995 the use of GST and DSG products fell sharply to be replaced mainly by LNG 150 µg + EE 30 µg. However the use of norethisterone (NRT) based products increased from 7.5% of the total to nearly 12% in 1997.
Figure 1.
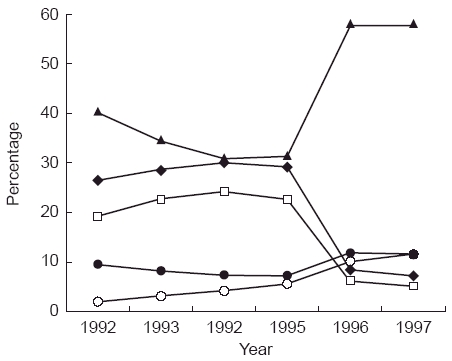
Percentage of total COC use by progestogen component.
Cases
Two hundred and ninety-six cases were identified that met the inclusion criteria and were not in any exclusion category. Amongst them there were nine fatalities. The overall incidence rate between 1992 and 1997 was 3.8 per 10 000 women years of exposure. The crude incidence rate for LNG 150 µg and EE 30 µg was 3.4 per 10 000 exposed woman years (EWY) (Table 2). The triphasic LNG formulation had a crude incidence rate of 2.5 per 10 000 and LNG 250 µg + EE 30 µg which produced only 8 cases, had a rate of 5.7 per 10 000. The rates for GST 75 µg and EE 30 µg and DSG 150 µg and EE 30 µg were similar, 4.4 and 4.3, respectively. However, the low oestrogen DSG product had a higher crude incidence rate (4.8 per 10 000). There were 2.8 cases per 10 000 EWY amongst 15–19-year-olds, the rate amongst 20–24-year-olds was slightly lower at 2.5. The incidence of VTE increased markedly with age, with a rate of 16.7 per 10 000 amongst women aged 45–49 years.
Nested case-control studies
The characteristics of the cases and controls in the two studies are shown in Table 3. The numbers of cases in the year-of-birth-matched study are lower than those in the 5-year-banded study, as there were fewer exposed controls available – notably for older women. One case was orphaned (no controls could be identified) in the 5-year-banded study and a further 10 in the exact age matched study. Most of these cases were over the age of 40 years. A larger proportion of cases than controls had BMIs over 30 kg m−2 and were current smokers. The only chronic disease where there were significant differences between cases and controls was asthma. More cases than controls had asthma and because of this finding, the number of non-OC prescriptions was recalculated to exclude treatments for asthma. Non-OC/nonasthma prescriptions in the 6 months preceding the event date were used as a proxy measure for general ill health. The proportion of women with no such prescriptions was higher amongst controls than among cases.
Table 3.
Characteristics of cases and controls.
| Year of birth matched controls | 5 years banded controls | |||
|---|---|---|---|---|
| Cases (%) | Controls (%) | Cases (%) | Controls (%) | |
| BMI category (kg/m2) | ||||
| < 20 | 26 (9.2) | 155 (14.1) | 28 (9.6) | 158 (13.5) |
| 20–24.99 | 110 (38.6) | 537 (48.9) | 115 (38.9) | 540 (46.1) |
| 25–29.99 | 54 (19.0) | 164 (14.9) | 56 (18.9) | 209 (17.8) |
| 30–34.99 | 25 (8.8) | 62 (5.7) | 25 (8.5) | 53 (4.5) |
| 35 and over | 19 (6.7) | 20 (1.8) | 19 (6.4) | 34 (2.9) |
| Unknown | 51 (17.9) | 160 (14.6) | 53 (17.9) | 178 (15.2) |
| Significance | Chi2 = 35.75; d.f. = 5; P < 0.001 | Chi2 = 21.99; d.f. = 5; P < 0.001 | ||
| Smoking | ||||
| Non smoker | 141 (49.5) | 712 (64.9) | 150 (50.7) | 741 (63.2) |
| Smoker | 102 (35.6) | 302 (27.5) | 102 (34.5) | 347 (29.6) |
| Unknown | 42 (14.7) | v84 (7.7) | 44 (14.9) | 84 (7.2) |
| Significance | Chi2 26.46; d.f. = 2; P < 0.001 | Chi2 = 24.01; d.f. = 2; P < 0.001 | ||
| Asthma | ||||
| Absent | 231 (81.1) | 987 (89.9) | 240 (82.1) | 1084 (92.5) |
| Present | 54 (19.0) | 111 (10.1) | 56 (18.9) | 88 (7.5) |
| Significance | Chi2 = 16.82; d.f. = 1; P < 0.001 | Chi2 = 34.78; d.f. = 1; P < 0.001 | ||
| Duration of exposure | ||||
| Under 6 months | 114 (42.1) | 344 (32.9) | 121 (42.9) | 401 (36.0) |
| 6–11.99 months | 46 (17.0) | 211 (20.2) | 47 (16.7) | 223 (20.0) |
| 12 months and more | 111 (41.0) | 491 (47.0) | 114 (40.4) | 489 (43.9) |
| Significance | Chi2 = 8.03; d.f. = 2; P < 0.05 | Chi2 = 4.81; d.f. = 2; P = 0.090 | ||
| Diastolic blood pressure | ||||
| Under 90 mmHg | 243 (85.3) | 1012 (92.2) | 249 (84.1) | 1069 (91.2) |
| 90 mmHg and over | 17 (6.0) | 34 (3.1) | 18 (6.1) | 48 (4.1) |
| No record | 25 (8.8) | 52 (4.7) | 29 (9.8) | 55 (4.7) |
| Significance | Chi2 = 12.86; d.f. = 2; P < 0.05 | Chi2 = 14.14; d.f. = 2; P = 0.001 | ||
| Number of non-OC and nonasthma treatment prescriptions in 6 months prior to event | ||||
| None | 60 (21.1) | 397 (36.2) | 62 (21.0) | 434 (37.0) |
| 1–2 | 86 (30.2) | 370 (33.7) | 92 (31.1) | 388 (33.1) |
| 3 and more | 139 (48.8) | 331 (30.2) | 142 (48.0) | 350 (29.9) |
| Significance | Chi2 = 39.57; d.f. = 2; P < 0.001 | Chi2 = 41.52; d.f. = 2; P < 0.001 | ||
The odds ratios (ORs) with 95% confidence intervals for the individual OC formulations, unadjusted and adjusted for BMI category, smoking status, diastolic blood pressure, asthma, duration of COC exposure and non-OC/nonasthma prescriptions are shown in Table 4. Adjustment had little effect on the odds ratios for most of the COC formulations. The ORs in the 5-year-banded study are consistently higher than those derived from the exact-year-of-birth matched study for the newer products (DSG + 30 µg EE, DSG + 20 µg EE, GST + 30 µg EE and NRG + 35 µg EE). The OR for the sequential LNG product is lower in the 5-year-banded study than the year-of-birth-matched study. There is no significant difference between the products in the year-of-birth-matched study, but using the 5-year-banded study there is a statistically significant increase associated with CPA + 35 µg EE.
Table 4.
Odds ratios crude and adjusted by COC product.
| Product | Year of birth controls | 5 years banded controls | ||||||
|---|---|---|---|---|---|---|---|---|
| Cases | Controls | Unadjusted | Adjusted | Cases | Controls | Unadjusted | Adjusted | |
| LNG 150 µg + Ethinyloestradiol 30 µg | 62 | 224 | REFERENCE | 64 | 273 | REFERENCE | ||
| 1.4 (0.9,2.1) | 1.4 (0.9,2.1) | |||||||
| DSG 150 µg + Ethinyloestradiol 30 µg | 62 | 218 | 1.1 (0.7,1.7) | 1.0 (0.6,1.6) | 65 | 223 | 1.4 (0.9,2.2) | 1.5 (0.9,2.3) |
| GST 75 µg + Ethinyloestradiol 30 µg | 60 | 204 | 1.1 (0.7,1.7) | 1.3 (0.8,2.1) | 63 | 208 | 1.1 (0.6,2.1) | 1.0 (0.5,2.0) |
| DSG 150 µg + Ethinyloestradiol 20 µg | 17 | 76 | 0.8 (0.4,1.6) | 0.8 (0.4,1.6) | 18 | 76 | 0.6 (0.4,1.1) | 0.6 (0.4,1.0) |
| Triphasic LNG (50 75 125) + Ethinyloestradiol (30 40 30)µg | 25 | 135 | 0.7 (0.4,1.1) | 0.7 (0.4,1.2) | 26 | 178 | 1.6 (0.8,3.1) | 1.6 (0.8,3 3.2) |
| NRG 250 µg + Ethinyloestradiol 35 µg | 15 | 51 | 1.1 (0.6,2.1) | 1.1 (0.6,2.3) | 15 | 45 | 0.7 (0.2,1.8) | 0.8 (0.3,2.1) |
| NRT 500 µg + Ethinyloestradiol 35 | 4 | 42 | 0.3 (0.1,0.9) | 0.3 (0.1,1.0) | 5 | 34 | 2.8 (1.4,5.6) | 2.3 (1.1, 4.7) |
| Cyproterone acetate 2 mg Ethinyloestradiol 35 µg | 16 | 36 | 1.7 (0.9,3.3) | 1.4 (0.7,2.9) | 16 | 26 | 0.8 (0.5,1.3) | 0.9 (0.5,1.4) |
| BMI < 20 | 26 | 155 | 0.8 (0.5,1.3) | 0.7 (0.5,1.2) | 28 | 158 | 1.3 (0.8,1.8) | 1.2 (0.8,1.8) |
| BMI 20–25 | 110 | 537 | REFERENCE | 115 | 540 | REFERENCE | ||
| BMI 25–30 | 54 | 164 | 1.6 (1.1,2.4) | 1.6 (1.1,2.3) | 56 | 209 | 1.3 (0.8,1.8) | 1.2 (0.8,1.8) |
| BMI 30–35 | 25 | 62 | 2.0 (1.2,3.3) | 1.9 (1.1,3.2) | 25 | 53 | 2.1 (1.2,3.6) | 2.0 (1.1,3.4) |
| BMI 35 + | 19 | 20 | 4.8 (2.5,9.3) | 3.8 (1.8,8.0) | 19 | 34 | 2.6 (1.4,4.7) | 2.0 (1.0,3.9) |
| Unknown BMI | 51 | 160 | 1.7 (1.1,2.5) | 1.1 (0.7,1.8) | 53 | 178 | 1.5 (1.0,2.2) | 1.0 (0.6,1.7) |
| No asthma | 231 | 987 | REFERENCE | 240 | 1084 | REFERENCE | ||
| Asthma | 54 | 111 | 2.2 (1.5,3.2) | 1.9 (1.3,2.9) | 56 | 88 | 3.1 (2.1,4.5) | 2.5 (1.6,3.8) |
| Non smoker | 141 | 712 | REFERENCE | 150 | 741 | REFERENCE | ||
| Smoker | 102 | 302 | 1.8 (1.4,2.5) | 2.0 (1.4,2.7) | 102 | 347 | 1.5 (1.1,2.0) | 1.5 (1.1,2.1) |
| Smoking unknown | 42 | 84 | 3.1 (1.9,5.0) | 2.9 (1.7,5.1) | 44 | 84 | 3.1 (2.0,4.9) | 3.0 (1.7,5.9) |
| Diastolic BP < 90 mmHg | 243 | 1012 | REFERENCE | 249 | 1069 | REFERENCE | ||
| Diastolic BP 90 mmHg and over | 17 | 34 | 2.0 (1.0,3.7) | 1.1 (0.5,2.3) | 18 | 48 | 1.6 (0.9,2.9) | 1.3 (0.7,2.6) |
| BP unknown | 25 | 52 | 2.3 (1.3,3.9) | 1.5 (0.8,2.9) | 29 | 55 | 2.5 (1.5,4.2) | 1.7 (0.9,3.2) |
| 0 non-OC scripts | 60 | 397 | REFERENCE | 62 | 434 | REFERENCE | ||
| 1–2 non-OC scripts | 86 | 370 | 1.5 (1.0,2.3) | 1.6 (1.0,2.5) | 92 | 388 | 1.6 (1.2,2.3) | 1.6 (1.1,2.3) |
| 3 and more non-OC scripts | 139 | 331 | 2.5 (1.8,3.5) | 2.2 (1.7,3.7) | 142 | 350 | 2.9 (2.1,4.0) | 2.3 (1.6,3.3) |
| Exposure < 6months | 120 | 373 | 1.4 (1.1,1.9) | 1.3 (1.0,1.8) | 127 | 432 | 1.3 (1.0,1.7) | 1.1 (0.8,1.5) |
| Exposure 6 months or more | 165 | 725 | REFERENCE | 169 | 740 | REFERENCE | ||
Note: statistically significant ORs(at the 5% level) are shown in bold.
With the exception of BMI, there was little difference between the estimated odds ratios for the confounding variables generated by the two studies. The year of birth matched controls resulted in a stronger association between BMI and VTE than did the 5-year-banded controls.
Following postal enquiry, additional information was available on 181 women, and the diagnoses in 149 of the cases was verified either through secondary evidence provided by the general practitioner or by death certificates. For the other 28 cases there was no confirmatory evidence but the women had been treated on clinical grounds either with or without investigation. In order to assess the robustness of the data from the computer file we compared the ORs of the verified cases with all cases using the year-of-birth-matched study. LNG 150 µg + EE 30 µg was used as the reference category. The adjusted odds ratios for DSG 150 µg + EE 30 µg were 1.0 (0.5,2.0) and 1.0 (0.7, 1.7) for the verified and all cases, respectively, and for DSG 150 µg + EE 20 µg the ORs were 1.3 (0.6,3.1) and 0.8 (0.4,1.5). For GST 75 µg + EE 30 µg the ORs were 1.2 (0.6, 2.4) and 1.3 (0.8,2.0), and for triphasic LNG they were 0.6 (0.2,1.3) and 0.7 (0.4,1.2).
In order to compare our results with those reported by other investigators we computed the ORs for aggregated COCs containing different progestogens. In this analysis we focused on the year-of-birth-matched study and all LNG products with less than 50 µg EE as the reference category. We adjusted for the same confounding variables as described in the analysis of the COC formulations. The OR for DSG based products was 1.2 (0.8,1.7), for GST it was 1.4 (1.0,2.3), for NRT it was 0.8 (0.5,1.5) and for NRG 1.4 (0.7,2.7).
Finally we computed the ORs for both formulations and progestogens for cases that occurred before 1996, i.e. before the change in usage patterns consequent upon the CSM’s 1995 statement. The incidence rate of VTE amongst users of LNG between 1992 and 1995 was 3.6 per 10 000 EWY; between 1996 and 1997 it was 3.1 per 10 000 EWY. The pre1996 ORs are set out in Table 5.
Table 5.
Adjusted ORs using year of birth controls and 5 years banded controls restricted to cases that occurred before 1996.
| Year of birth controls | 5 years banded controls | |||
|---|---|---|---|---|
| Product formulation or progestogen | Adjusted OR | 95% CI | Adjusted OR | 95% CI |
| LNG 150 µg + EE 30 µg | REFERENCE | |||
| DSG 150 µg + EE 30 µg | 1.1 | 0.6, 1.6 | 1.4 | 0.9, 2.4 |
| GST 75 µg + EE 30 µg | 1.3 | 0.8, 2.1 | 1.6 | 1.0, 2.6 |
| DSG 150 µg + EE 20 µg | 0.9 | 0.4, 1.8 | 1.2 | 0.6, 2.4 |
| Triphasic LNG (50 75 125) + EE (30 40 30) µg | 0.6 | 0.3, 1.2 | 0.6 | 0.3, 1.0 |
| NRG 250 µg + EE 35 µg | 1.3 | 0.6, 2.9 | 2.1 | 0.9, 5.0 |
| Cyproterone acetate 2 mg + EE 35 µg | 1.1 | 0.5, 2.4 | 2.3 | 1.0, 5.5 |
| LNG | REFERENCE | 0.7,1.7 | 1.6 | 1.1, 2.4 |
| DSG | 1.1 | 0.7,1.7 | 1.6 | 1.1, 2.4 |
| GST | 1.5 | 0.9,2.3 | 1.8 | 1.2, 2.8 |
| NRG | 1.5 | 0.7,3.4 | 2.5 | 1.1, 5.6 |
Discussion
We report on a case-control study nested within a cohort investigation using the GPRD. The purpose of the cohort study was to estimate the crude incidence rates of idiopathic VTE amongst women exposed to different combined oral contraceptives. The robustness of this type of study is determined by the completeness with which the cases are identified within the cohort and the calculation of size and exposure of the cohort members. The nested case-control study was designed to produce a data set from which the risks associated with different formulations could be estimated taking account of potential confounding factors, such as age, BMI, etc. In a case-control study it is essential that controls are selected from the same population as the cases. One of the great advantages of a general practice database study is that the controls can be selected from the same practices as the cases. In this study we randomly selected controls from the same practices as the case in order to avoid bias in reporting, record keeping and the use of health services. The potential confounding relating to age, which affects both the underlying risk and a range of other factors, was removed by matching controls to cases according to the year of birth. The strong trends in patterns of utilization of oral contraceptives over time could affect the risk of exposure to a particular oral contraceptive. Therefore the controls were selected on the basis that they were using a COC on the day of the event in the case to which they were matched. Data were extracted from the database that were thought to be possible confounders to the estimate of risk. No observational study can control for every potential confounding factor. Database studies are limited to consideration of the material collected routinely by clinicians during the course of medial practice. The classical case-control study is limited to consideration of potential confounding variables that the investigators deemed relevant during the design phase and that they were able to collect during the course of the study. We make no claim that this study is free from uncontrolled confounding and/or bias.
Amongst the more important findings of this study are the increased risk of VTE associated with increasing age, high BMI (particularly BMI over 30 kg m−2), smoking, asthma and general health measured by the proxy of the number of prescriptions of non OC and non asthma indication. Most recent studies indicate an increased risk associated with smoking, but not all reach statistical significance, possibly because there were insufficient cases. Our findings are consistent with those of Petitti et al.[15] and the findings of other recent studies [2–4]. The association between high BMI and risk of VTE has been reported before but in this investigation the narrow categories serve to demonstrate the effect with greater clarity. It is noteworthy that about 9% of the women in both our studies had BMIs over 30. The increased risk associated with asthma has not been reported elsewhere and is worthy of further investigation.
The absolute incidence of VTE amongst current users of COCs found in this investigation is consistent with findings from most other studies [6, 7, 12]. Higher crude rates associated with the newer compared with older products reflects differences in the age of users, years of introduction and the effects of confounding variables. Neither the unadjusted nor the adjusted ORs for individual formulations differed significantly using either the year of birth matched controls or the 5-year banded controls. The 5-year-banded controls resulted in higher ORs for all of the major products except the triphasic LNG formulations. The only statistically significant increase in ‘risk’ was associated with cyproterone acetate (CPA) + 35 EE µg, OR 2.3 (95% CI 1.1, 4.8). CPA + 35 µg EE is an atypical product, although it acts as an oral contraceptive its indication is specifically for the treatment of acne. With this product the possibility of confounding by indication is high. We have shown that the incidence of VTE amongst women using COCs increases with age. However, it is unlikely that the changes in underlying risk within 5 year age bands would be great enough to affect the ORs. More important is that age is a proxy for a number of other variables that might affect exposure to a particular oral contraceptive or the underlying risk – such as prior pregnancy, duration of use and revelation to the prescriber of a family history of thromboembolic disease. Because of the relatively rapid changes in many of these factors especially at the younger end of the reproductive age range and the differences in patterns of OC use, even between women 1 year different in age, we believe that matching the controls by year of birth is more appropriate than matching within an age band. For the newer products the ORs derived from the pre1996 data tend to be higher than those from the complete data set.
When COCs are aggregated according to their progestogen component, there is a nonsignificant increase in risk associated with DSG, GST and NRG products (the so-called third generation products) when compared to LNG based formulations. There are two DSG based formulations, both are monophasic, one has 30 µg EE and the other has 20 µg. Over 95% of GST products used were the monophasic formulation. By contrast there are three low dose EE + LNG formulations – the monophasic product with 150 µg plus 30 µg EE is the most frequently used (61%), the triphasic preparation has 34% of overall use and the third (LNG 250 µg + EE 30 µg) represents about 5% of LNG use. These products are used by women with different characteristics. The users of the 150 µg monophasic formulation are younger than the users of the triphasic preparation. The 250 µg monophasic preparation is used by women who are older than those on any other preparation with an average age of 30 years and 23.6% of users being over 35-years-old. The OR for the triphasic preparation compared with the monophasic 150 µg preparation is less than 1. The use of all LNG products as the reference population rather than a specific formulation lowers the baseline risk and therefore increases the ORs of the other formulations aggregated on the basis of their progestogen component. It does not appear rational to aggregate these three different LNG COC formulations in view of the differences in their patterns of utilization and differences in the time of their introduction. We therefore believe that consideration of specific formulations gives more realistic estimates of their relative risks.
Because of the small numbers of cases no other study has compared individual products. We can only compare our progestogen aggregated findings with those of other investigators. The UK part of the WHO study using hospital controls, yielded ORs of 2.3 (1.1, 4.9) for DSG and 2.0 (0.8, 4.7) for GST [4]. The community controls resulted in ORs of 1.8 (0.7, 4.8) and 0.9 (0.3, 2.8) for DSG and GST, respectively. Thus the only significantly elevated risk was for DSG using hospital controls. All the odds ratios are consistent with our findings, that is our estimate is within the lower limits of their 95% confidence intervals. The WHO study was a hospital based case control study of women aged 20–44 years. Controls were matched to cases within 5 years age bands. It was carried out between 1989 and 1993 (very shortly after GST products were first marketed in the UK). The Transnational study [3] was similar in design to the WHO but was concerned with women aged between 16 and 44 years. The ORs for DSG and GST in the UK centres were 1.6 (1.0, 2.5) and 1.4 (0.9, 2.3), respectively. The investigation was carried out between 1993 and 1995. Our study is not entirely comparable with these two hospital based case-control studies, however, we were able to reanalyse our data in such a way as to simulate them. We used the 5-year-banded study, aggregated formulations according to their progestogen type, used the same three BMI and smoking categories in the adjustment and restricted the analysis to events that occurred before 1995. This yielded ORs of 1.6 (1.0, 2.7) for DSG and 1.8 (1.1, 3.0) for GST. Both ORs are consistent with those reported by the WHO and Transnational teams.
The comparison of the whole data set with the pre 1996 data is of interest. If the high ORs for VTE associated with the newer progestogens were due to an inherent characteristic of the products, then the estimated risk should be unaffected by changes in the pattern of usage. On the other had, if the results of the earlier studies were due to the characteristics of the users, then a change in OR might be expected following a major change in utilization patterns. The progestogen specific analysis shows the ORs for GST and NRG in the total data set are lower than those for the pre 1996 data. The OR for DSG is virtually unchanged (1.2 and 1.1) and neither is significant.
The disparity between our results and those reported by the Boston Collaborative Surveillance Project (BCDSP) are at first sight more difficult to understand. However, it is important to note that differences in the way the GPRD is analysed and the time period during which the studies were undertaken may lead to different results, and indeed may give rise to contrary conclusions, even though the database itself is a valid and important source for epidemiological research. In our study we used all the available data on the GPRD that was deemed to be of ‘research standard’ by the management of the GPRD. The BCDSP team restricted their investigation of mortality to 470 of the 618 qualifying practices; their investigation of morbidity, from which fatalities were excluded, was restricted to 365 practices. Another important difference between the two studies is in the way in which potential cases were identified. In this study, women with a recorded relevant diagnosis, evidence of anticoagulant treatment, who could have been using a combined oral contraceptive on the day of the event and who had none of the exclusion factors, qualified for consideration as cases. The BCDSP team appears to have used a coded entry designating hospital admission as a primary search criteria. We did not use this as a primary search criteria because many of the emergency admissions not initiated by the GP were not coded. As part of our study we undertook a verification exercise but restricted this to women who were still registered with their practice. The verification exercise demonstrated that the majority of cases were confirmed by objective tests (e.g. Doppler ultrasound, venogram, etc.) and all except two had been admitted to hospital, even though in many there was no coded record of the hospital admission in the computer record. We took the view that the probability of anticoagulant therapy being initiated and maintained without the diagnosis of VTE either being confirmed or there being convincing clinical evidence in support of it was so slight that it could be discounted. Had a coded record of hospital admission been a case-selection requirement in our study, there would have been considerable under-identification of cases. The verification study confirmed our view. The BCDSP study differed from our study and all the other studies in as much as it was restricted to women using combined oral contraceptives containing either LNG or GST or DSG. This affects both the case and control qualification criteria and thus their study is not directly comparable with our own or the other studies. Finally, their study was restricted to women under the age of 40 whereas we considered women up to the age of 49 years.
The crude incidence of idiopathic VTE reported by the BCDSP [2] was about half that found in our study. The only other studies to report such low incidences were based on the Puget Sound Group Health Cooperative Database [16, 17] and were cited as supportive of the credibility of the estimates. Both of those studies were undertaken by the BCDSP. In the first [16] the rate of 1.9 cases per 10 000 women years of exposure was calculated from 7 exposed cases in 36 428 women years of exposure. The second study [17] has 3 exposed cases of venous thromboembolism amongst 37 807 women years of exposure resulting in an incidence rate amongst users of 0.8 per 10 000 women years of exposure. Both of these estimates are substantially lower than rates derived from other and larger studies. For example, Gersman et al.[18], reported a rate of 4.2 per 10 000 exposed women years of exposure to COCs containing less than 50 µg oestrogen from a study on the Michigan Medicaid population; it was calculated from 53 exposed cases of idiopathic VTE amongst 127 000 women years of exposure. An analysis of the UK MediPlus database resulted in a rate of 4.1 per 10 000 EWY (83 cases in 202517 EWY). The rate estimated from the present study, 3.8 per 10 000 EWY (289 cases) is in line with the majority of previous findings, that of the BCDSP −2.3 per 10 000 EWY is not. It is of particular interest that the rate of VTE amongst the users of LNG based COCs with less than 50 µg of oestrogen in the BCDSP study is 1.6 per 10 000 EWY. The differences between the two rates could be due to differences in case identification, or the way in which the denominator was estimated or differences in the inclusion and exclusion criteria or a combination of all of these.
One of the principle objectives of the present study was to compare the risks associated with different formulations rather than compare the risks between formulations aggregated according to their progestogen component and in this respect the study differs from that carried out by the BCDSP [2]. Nevertheless we did make a comparison between formulations aggregated by progestogen and found no difference in risk of idiopathic VTE between any of the newer progestogens (DSG, GST and NRG) and LNG. The BCDSP study showed ORs of around 2 for both DSG and GST. Within the design of the nested case-control study the main differences between this investigation and the BCDSP, other than the way cases were identified and the numbers of practices involved, were as follows: i) we included exposure to any low dose COC whereas the BCDSP restricted their investigation to women using LNG, DSG or GST based COCs, ii) we included fatalities, whereas the BCDSP excluded them, iii) in the first of our nested case control studies we matched by year of birth, whereas the BCDSP matched within 2 years of age, iv) our study used cases that accrued between 1992 and mid-1997, rather than those from 1991 to November 1994. In addition, the classification of some of the potential confounding variables differed.
Following their initial publication, the BCDSP team published a re-analysis of their data in which they matched by year of birth [19]. This was achieved by omitting controls that were not born in the same year as the cases with which they were associated rather than identifying a new set of controls or substituting new controls for those lost. The OR for both DSG and GST remained at around 2 compared with LNG based COCs.
The inclusion of fatalities materially affected our results as there were proportionately more fatalities amongst the LNG group than either of the others.
We reanalysed our data to emulate the BCDSP case control analysis as far as possible, thus we restricted the analysis to cases that occurred between 1992 and 1994, restricted both cases and controls to women using LNG or GST or DSG based COCs, omitted deaths, restricted the analysis to women under 40 and used the same three categories for BMI and smoking. With these restrictions, our data yielded ORs of 1.6 (0.8,3.3) and 1.3 (0.5,3.4) for DSG and GST, respectively, in the 1-year-band matched study.
In our study the ORs for individual products did not differ significantly when cases were compared with the exact year-of-birth-matched controls. On the basis of the current analysis we do not believe there are differences in risk between COCs. However, we have identified high BMI, smoking, asthma and concurrent ill health as factors that increase the risk of VTE in women. These factors should be taken into account by prescribers as they are more likely to affect the overall risk of VTE than the use of any one or other of the low oestrogen COCs.
Acknowledgments
This project was overseen by a Scientific Advisory Board which had full access to the data, commented on the methodology at all stages and advised on the analysis. The members of the board were as follows: Professor Jacques LeLorier (University of Montreal), Professor Lynn Rosenberg (University of Boston), Professor John Newton (University of Birmingham), Professor John Guillebaud (University of London), Professor Thomas MacDonald (University of Dundee), Dr Alan Dean (EPIC) and Dr Sam Rowlands (General Practitioner).
This study was funded by an unconditional grant from NV Organon and Schering AG.
References
- 1.Committee on the Safety of Medicines. Combined oral contraceptives and thromboembolism. London: Committee on the Safety of Medicines; 1995. [Google Scholar]
- 2.Jick H, Jick SS, Gurewich V, Myers MW, Vasilakis C. Risk of idiopathic cardiovascular death and nonfatal venous thromboembolism in women using oral contraceptives with differing progestogen components. Lancet. 1995;346:1589–1593. doi: 10.1016/s0140-6736(95)91928-7. [DOI] [PubMed] [Google Scholar]
- 3.Spitzer WO, Lewis MA, Heinemann LA, Thorogood M, MacRae KD. Third generation oral contraceptives and risk of venous thromboembolic disorders: an international case-control study. Transnational Research Group on Oral Contraceptives and the Health of Young Women. Br Med J. 1996;312:83–88. doi: 10.1136/bmj.312.7023.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.World Health Organization Collaborative study of cardiovascular disease and steroid hormone contraception. Effect of different progestagens in low oestrogen oral contraceptives on venous thromboembolic disease. Lancet. 1995;346:1582–1588. [PubMed] [Google Scholar]
- 5.Lewis MA, Heinemann LAJ, MacRae KD, Bruppacher R, Spitzer WO. The increased risk of venous thromboembolism and the use of third generation progestogens: role of bias in observational research. Contraception. 1996;54:5–13. doi: 10.1016/0010-7824(96)00112-6. [DOI] [PubMed] [Google Scholar]
- 6.Gerstman BB, Piper JM, Tomita DK, Ferguson WJ, Stadel BV, Lundin FE. Oral contraceptive estrogen dose and the risk of deep venous thromboembolic disease. Am J Epidemiology. 1991;133:32–37. doi: 10.1093/oxfordjournals.aje.a115799. [DOI] [PubMed] [Google Scholar]
- 7.Vessey M, Mant D, Smith A, Yeates D. Oral contraceptives and venous thromboembolism: findings in a large prospective study. Br Med J Clin Res Ed. 1986;292:526. doi: 10.1136/bmj.292.6519.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Helmrich SP, Rosenberg L, Kaufman DW, Strom B, Shapiro S. Venous thromboembolism in relation to oral contraceptive use. Obstet Gynecol. 1987;69:91–95. [PubMed] [Google Scholar]
- 9.Guillebaud J. Advising women on which pill to take: the informed user should be the chooser. Br Med J. 1995;311:1111–1112. doi: 10.1136/bmj.311.7013.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Editorial. Whipping up panic about the pill. Nature. 1995;377:663. doi: 10.1038/377663a0. [DOI] [PubMed] [Google Scholar]
- 11.Weiss N. Third-generation oral contraceptives: how risky? Lancet. 1995;346:1570. doi: 10.1016/s0140-6736(95)91921-x. [DOI] [PubMed] [Google Scholar]
- 12.Farmer RDT, Lawrenson RA, Thompson CR, Kennedy JG, Hambleton IR. Population based study of risk of venous thromboembolism associated with various oral contraceptives. Lancet. 1997;349:83–88. doi: 10.1016/s0140-6736(96)07496-x. [DOI] [PubMed] [Google Scholar]
- 13.Jick H, Jick SS, Derby LE. Validation of information recorded on general practitioner based computerised data resource in the United Kingdom. Br Med J. 1991;302:766–768. doi: 10.1136/bmj.302.6779.766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Van Staa T, Abenheim L. The quality of information on a UK Database of Primary Care records: a study of hospitalizations due to hypoglycemia and other conditions. Pharmacoepidemiol Drug Safety. 1994;3:15–21. [Google Scholar]
- 15.Petitti DB, Wingerd J, Pellegrin F, Ramcharan S. Oral contraceptives, smoking, and other factors in relation to risk of venous thromboembolic disease. Am J Epidemiology. 1978;108:480–485. doi: 10.1093/oxfordjournals.aje.a112646. [DOI] [PubMed] [Google Scholar]
- 16.Porter JB, Hunter JR, Danileson DA, Jick H, Stergachis A. Oral contraceptives and nonfatal vascular disease – Recent experience. Obstetrics Gynaecol. 1982;59:299–302. [PubMed] [Google Scholar]
- 17.Porter JB, Hunter JR, Jick H, Stergachis A. Oral contraceptives and nonfatal vascular disease. Obstetrics Gynaecol. 1985;66:1–4. [PubMed] [Google Scholar]
- 18.Gerstman BB, Piper JM, Freiman JP, et al. Oral contraceptive oestrogen and progestin potencies and the incidence of deep vein thromboembolism. Int Epidemiol. 1990;19:931. doi: 10.1093/ije/19.4.931. [DOI] [PubMed] [Google Scholar]
- 19.Jick H, Jick SS, Myers MW, Vasilakis C. Third generation oral contraceptives and venous thrombosis. (Letter) Lancet. 1997;349:742. doi: 10.1016/S0140-6736(05)60173-0. [DOI] [PubMed] [Google Scholar]


