Abstract
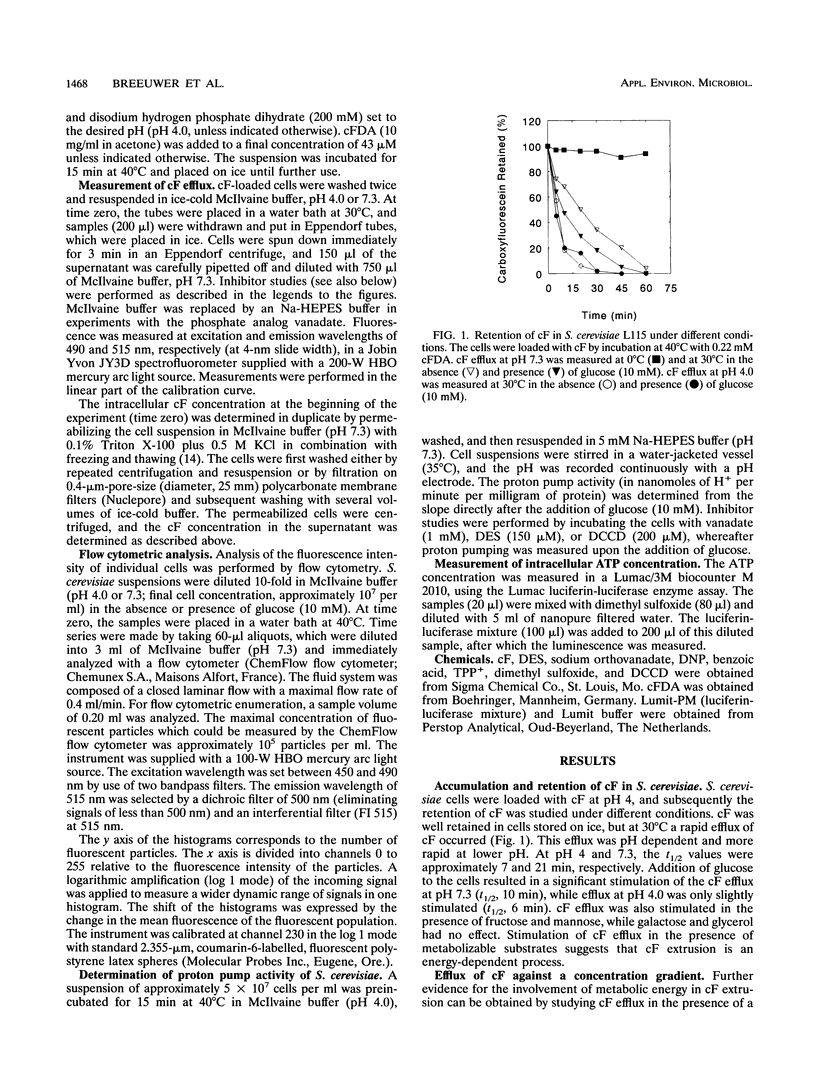
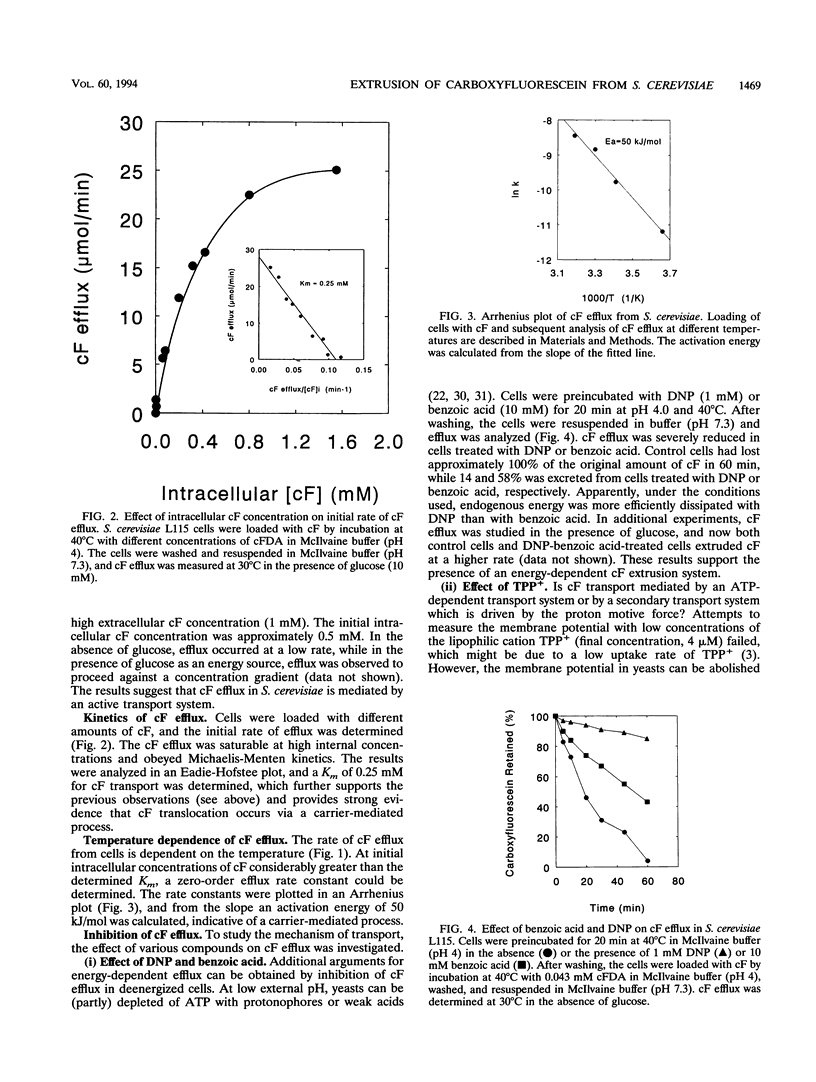
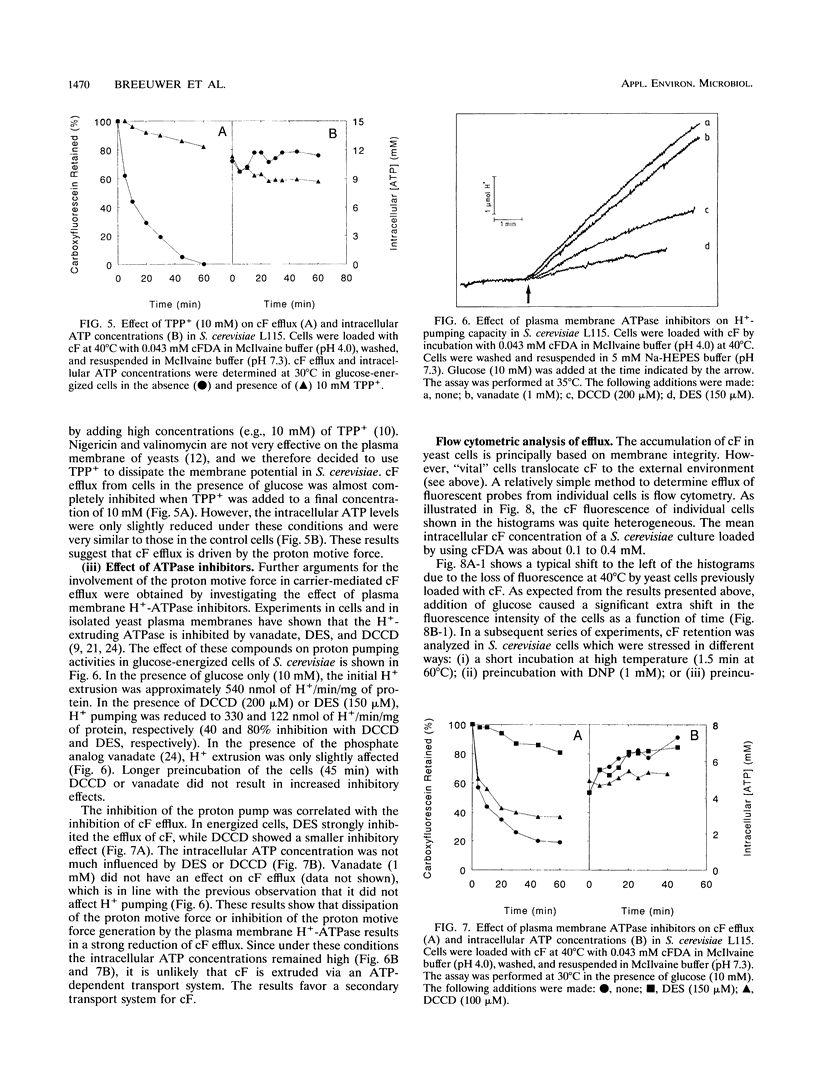
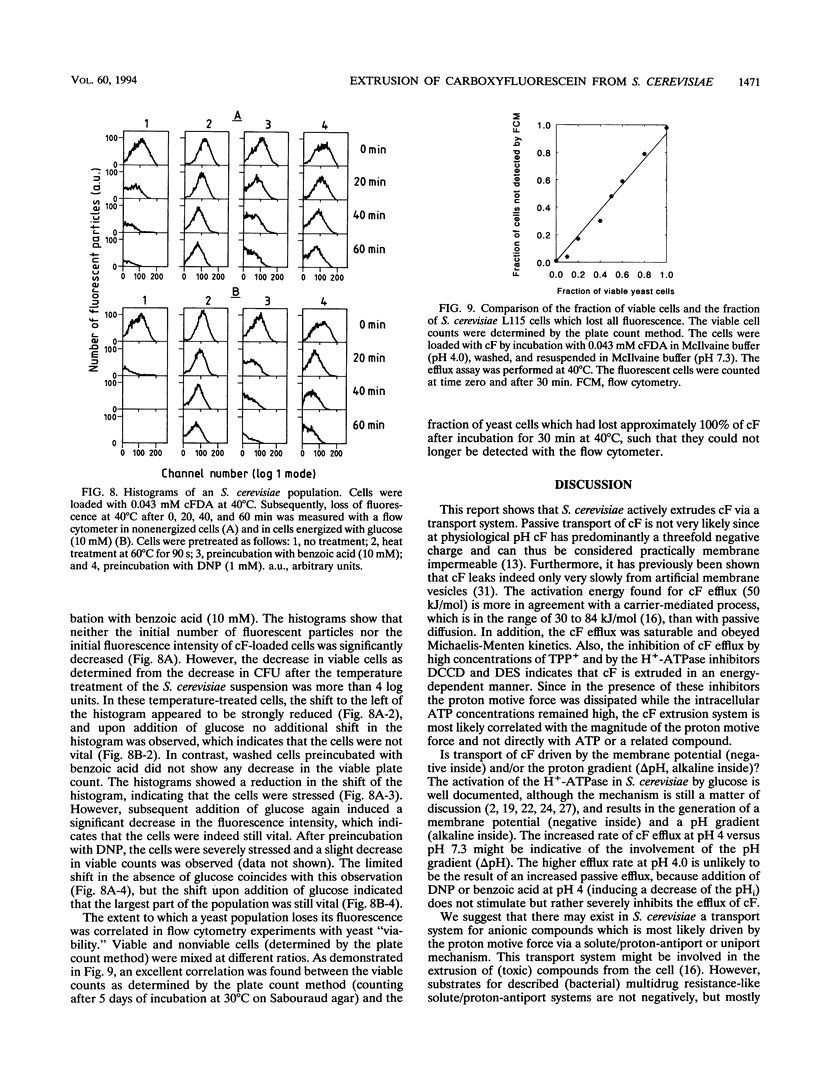
Carboxyfluorescein diacetate is a nonfluorescent compound which can be used in combination with flow cytometry for vital staining of yeasts and bacteria. The basis of this method is the assumption that, once inside the cell, carboxyfluorescein diacetate is hydrolyzed by nonspecific esterases to produce the fluorescent carboxyfluorescein (cF). cF is retained by cells with intact membranes (viable cells) and lost by cells with damaged membranes. In this report, we show that Saccharomyces cerevisiae extrudes cF in an energy-dependent manner. This efflux was studied in detail, and several indications that a transport system is involved were found. Efflux of cF was stimulated by the addition of glucose and displayed Michaelis-Menten kinetics. A Km for cF transport of 0.25 mM could be determined. The transport of cF was inhibited by the plasma membrane H(+)-ATPase inhibitors N,N'-dicyclohexylcarbodiimide and diethylstilbestrol and by high concentrations of tetraphenylphosphonium ions. These treatments resulted in a dissipation of the proton motive force, whereas the intracellular ATP concentration remained high. Transport of cF is therefore most probably driven by the membrane potential and/or the pH gradient. The viability of S. cerevisiae was determined by a two-step procedure consisting of loading the cells with cF followed by incubation at 40 degrees C in the presence of glucose. Subsequently, the fluorescence intensity of the cells was analyzed by flow cytometry. The efflux experiments showed an excellent correlation between the viability of S. cerevisiae cells and the ability to translocate cF. This method should prove of general utility for the rapid assessment of yeast vitality and viability.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen C. N., Harpur E. S., Gray T. J., Simmons N. L., Hirst B. H. Efflux of bis-carboxyethyl-carboxyfluorescein (BCECF) by a novel ATP-dependent transport mechanism in epithelial cells. Biochem Biophys Res Commun. 1990 Oct 15;172(1):262–267. doi: 10.1016/s0006-291x(05)80203-7. [DOI] [PubMed] [Google Scholar]
- Blanpain J. P., Ronjat M., Supply P., Dufour J. P., Goffeau A., Dupont Y. The yeast plasma membrane H(+)-ATPase. An essential change of conformation triggered by H+. J Biol Chem. 1992 Feb 25;267(6):3735–3740. [PubMed] [Google Scholar]
- Boxman A. W., Barts P. W., Borst-Pauwels G. W. Some characteristics of tetraphenylphosphonium uptake into Saccharomyces cerevisiae. Biochim Biophys Acta. 1982 Mar 23;686(1):13–18. doi: 10.1016/0005-2736(82)90146-8. [DOI] [PubMed] [Google Scholar]
- Bruning J. W., Kardol M. J., Arentzen R. Carboxyfluorescein fluorochromasia assays. I. Non-radioactively labeled cell mediated lympholysis. J Immunol Methods. 1980;33(1):33–44. doi: 10.1016/0022-1759(80)90080-0. [DOI] [PubMed] [Google Scholar]
- Diaper J. P., Tither K., Edwards C. Rapid assessment of bacterial viability by flow cytometry. Appl Microbiol Biotechnol. 1992 Nov;38(2):268–272. doi: 10.1007/BF00174481. [DOI] [PubMed] [Google Scholar]
- Goffeau A., Slayman C. W. The proton-translocating ATPase of the fungal plasma membrane. Biochim Biophys Acta. 1981 Dec 30;639(3-4):197–223. doi: 10.1016/0304-4173(81)90010-0. [DOI] [PubMed] [Google Scholar]
- Kovác L., Böhmerová E., Butko P. Ionophores and intact cells. I. Valinomycin and nigericin act preferentially on mitochondria and not on the plasma membrane of Saccharomyces cerevisiae. Biochim Biophys Acta. 1982 Dec 30;721(4):341–348. doi: 10.1016/0167-4889(82)90088-x. [DOI] [PubMed] [Google Scholar]
- Miozzari G. F., Niederberger P., Hütter R. Permeabilization of microorganisms by Triton X-100. Anal Biochem. 1978 Oct 1;90(1):220–233. doi: 10.1016/0003-2697(78)90026-x. [DOI] [PubMed] [Google Scholar]
- Molenaar D., Abee T., Konings W. N. Continuous measurement of the cytoplasmic pH in Lactococcus lactis with a fluorescent pH indicator. Biochim Biophys Acta. 1991 Nov 14;1115(1):75–83. doi: 10.1016/0304-4165(91)90014-8. [DOI] [PubMed] [Google Scholar]
- Molenaar D., Bolhuis H., Abee T., Poolman B., Konings W. N. The efflux of a fluorescent probe is catalyzed by an ATP-driven extrusion system in Lactococcus lactis. J Bacteriol. 1992 May;174(10):3118–3124. doi: 10.1128/jb.174.10.3118-3124.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neyfakh A. A., Bidnenko V. E., Chen L. B. Efflux-mediated multidrug resistance in Bacillus subtilis: similarities and dissimilarities with the mammalian system. Proc Natl Acad Sci U S A. 1991 Jun 1;88(11):4781–4785. doi: 10.1073/pnas.88.11.4781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prosperi E. Intracellular turnover of fluorescein diacetate. Influence of membrane ionic gradients on fluorescein efflux. Histochem J. 1990 Apr;22(4):227–233. doi: 10.1007/BF02386009. [DOI] [PubMed] [Google Scholar]
- Ramos S., Balbín M., Raposo M., Valle E., Pardo L. A. The mechanism of intracellular acidification induced by glucose in Saccharomyces cerevisiae. J Gen Microbiol. 1989 Sep;135(9):2413–2422. doi: 10.1099/00221287-135-9-2413. [DOI] [PubMed] [Google Scholar]
- Rotman B., Papermaster B. W. Membrane properties of living mammalian cells as studied by enzymatic hydrolysis of fluorogenic esters. Proc Natl Acad Sci U S A. 1966 Jan;55(1):134–141. doi: 10.1073/pnas.55.1.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano R. Effect of ATPase inhibitors on the proton pump of respiratory-deficient yeast. Eur J Biochem. 1980 Apr;105(2):419–424. doi: 10.1111/j.1432-1033.1980.tb04516.x. [DOI] [PubMed] [Google Scholar]
- Serrano R. In vivo glucose activation of the yeast plasma membrane ATPase. FEBS Lett. 1983 May 30;156(1):11–14. doi: 10.1016/0014-5793(83)80237-3. [DOI] [PubMed] [Google Scholar]
- Sigler K., Höfer M. Mechanisms of acid extrusion in yeast. Biochim Biophys Acta. 1991 Dec 12;1071(4):375–391. doi: 10.1016/0304-4157(91)90003-f. [DOI] [PubMed] [Google Scholar]
- Sychrová H., Kotyk A. Conditions of activation of yeast plasma membrane ATPase. FEBS Lett. 1985 Apr 8;183(1):21–24. doi: 10.1016/0014-5793(85)80945-5. [DOI] [PubMed] [Google Scholar]
- Talbot D., Shenton B. K., Givan A. L., Proud G., Taylor R. M. A rapid, objective method for the detection of lymphocytotoxic antibodies using flow cytometry. J Immunol Methods. 1987 May 4;99(1):137–140. doi: 10.1016/0022-1759(87)90042-1. [DOI] [PubMed] [Google Scholar]
- Verduyn C., Postma E., Scheffers W. A., Van Dijken J. P. Effect of benzoic acid on metabolic fluxes in yeasts: a continuous-culture study on the regulation of respiration and alcoholic fermentation. Yeast. 1992 Jul;8(7):501–517. doi: 10.1002/yea.320080703. [DOI] [PubMed] [Google Scholar]
- Warth A. D. Effect of benzoic acid on glycolytic metabolite levels and intracellular pH in Saccharomyces cerevisiae. Appl Environ Microbiol. 1991 Dec;57(12):3415–3417. doi: 10.1128/aem.57.12.3415-3417.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein J. N., Blumenthal R., Klausner R. D. Carboxyfluorescein leakage assay for lipoprotein-liposome interaction. Methods Enzymol. 1986;128:657–668. doi: 10.1016/0076-6879(86)28098-2. [DOI] [PubMed] [Google Scholar]



