Abstract
Aims
To test the association between reporting rates for sparfloxacin-induced phototoxicity and sunlight u.v. exposure, and the effects of regulatory action.
Methods
The reporting rates for phototoxicity with sparfloxacin to the French Pharmacovigilance System or to the Drug Manufacturer were compared with concurrent national mean u.v. exposure obtained from Météo-France, before and after the regulatory restrictions and warnings.
Results
There were 371 severe phototoxic reaction reports during the first 9 months of marketing (reporting rate of 0.4 per thousand treated patients), approximately four to 25 times that reported for other fluoroquinolones. The reporting rate correlated highly (r = 0.873, P < 0.001) with the mean monthly u.v. exposure from sunlight (from Météo-France). Regulatory action including warnings for physicians, and restricted indications dramatically decreased the number of reports, but not the reporting rate.
Conclusions
This is the first demonstration of a strong association between sunlight exposure in a population and drug-induced phototoxicity. Regulatory action had no effect on the reporting rate (individual exposed patient risk), though it solved the public health issue.
Keywords: phototoxicity, sparfloxacin, sunshine
Introduction
Sparfloxacin is a fluoroquinolone antibiotic that is indicated for community-acquired pneumonia, infective exacerbations of chronic obstructive lung disease and purulent sinusitis. The drug was launched in France in September 1994. From previous experience with nalidixic acid [1] and the other fluoroquinolones [2–4], the phototoxic potential of these drugs was known. However, by March 1995, the number of spontaneous reports of phototoxicity involving sparfloxacin appeared to be higher than expected. In order to assess the risk of phototoxicity associated with sparfloxacin and to obtain a better description of the toxicity (seriousness, severity, risk factors), a National Pharmacovigilance enquiry was set up by the French Medicines Agency in April 1995. Additionally, sunlight exposure data was obtained from the national weather service.
Methods
All cases of phototoxicity reported to the French Pharmacovigilance System or to the Marketing Authorization Holder (Spécia-Rhône-Poulenc-Rorer) from launch (September 1994) to July 1996 were analysed according to standard procedures [5]. The enquiry was coordinated by the Regional Pharmacovigilance Centre in Nancy. The index dates for reactions were dates of occurence of the reaction.
The reaction reporting rate was computed with reference to the estimated number of patients treated, derived from drug sales, an estimated daily dose (220 mg day−1) and treatment duration (7.4 days), the latter two obtained from prescription panels.
The reporting rates were compared with those obtained for other fluoroquinolones, during a previous national pharmacovigilance enquiry covering all fluoroquinolones [6], using the same methodology.
Mean monthly horizontal solar u.v. irradiation (u.v.) over the French territory (in J/cm2) was obtained from Météo France, the National Meteorological Service.
Statistical analysis was performed by χ2 analysis accepting P < 0.05 as significant.
Results
During the first 9 months of marketing (to July 1995), 371 cases of phototoxicity were reported. Photosensitivity or phototoxicity reactions represented 41% of all reported reactions and 98% of the skin reactions with sparfloxacin. The lesions were characterized by a solar erythema of the face and uncovered skin. Second-degree burns (blisters) occurred in almost 23% of cases. These lesions were associated with dysaesthesia of the hands in 13 cases, and visual disturbances in three cases, one of which had retinal oedema. The most unexpected clinical symptom was recurrent phototoxicity during renewed solar exposure, in some cases as late as 1 to 2 months after the drug had been stopped.
The patients (male:female ratio of 1 : 2) had a mean age of 43 years (range 11–83). The mean time between the start of the treatment and the onset of symptoms was 3 days (range 1–46).
The majority of patients (61%) recovered, while sequelae were observed in 11%; these included hyperpigmentation or scarring, especially in patients with second-degree burns. The most severe reactions were observed after high or moderate sunlight exposure. The outcome was unknown in 28% of cases.
The French Pharmacovigilance System received more reports of photosensitivity involving sparfloxacin than for any other fluoroquinolone (figures taken from another National enquiry) [6]. The reporting rate for phototoxicity was 40 per 100 000 treated patients for sparfloxacin (95% CI 36–44) vs 12 per 100 000 for lomefloxacin (95% CI 6.1–20.8), 5 per 100 000 for pefloxacin (95% CI 2.3–9.5), 0.1 per 100 000 for ciprofloxacin (95% CI 0.02–0.7) and norfloxacin (95% CI 0.02–0.34) and 0.3 per 100 000 for ofloxacin (95% CI 0.06–0.8).
Clinical symptoms observed with sparfloxacin were more severe than those reported or published with other fluoroquinolones, or for other phototoxic antibiotics like tetracyclines.
Following this Pharmacovigilance Enquiry, in June 1995 a series of measures were undertaken to reduce the risk of phototoxicity. The product information was altered to provide more information about the risk and severity of the lesions; skin exposure to sunlight and artificial u.v. was forbidden during treatment and for 3 days after its discontinuation. The indications were restricted to use only in community-acquired pneumonia and sinusitis where other antibiotics were felt to be inappropriate, i.e. in localities with a high prevalence of resistant pneumococci. This resulted in an immediate decrease in the number of patients treated monthly, from approximately 60–80 000 in April-May, to half that in June, to 3–5000 patients from July 1995 onwards.
In the year following this decision, between July 1995 and June 1996, only 11 cases of phototoxicity were reported in France. The estimated reporting rate for this period was 44 per 100 000 treated patients (95% CI 22–79).
The reporting rates varied with the mean horizontal solar u.v. radiation (Figure 1). After the regulatory decisions and changes in the SPC warning about the risk of phototoxicity in June 1995, the number of reports decreased dramatically but the relationship between the reporting rates and solar radiation remained evident. There was a significant linear correlation (r = 0.873, P < 0.001) between the reporting rates and solar u.v. radiation (Figure 2).
Figure 1.
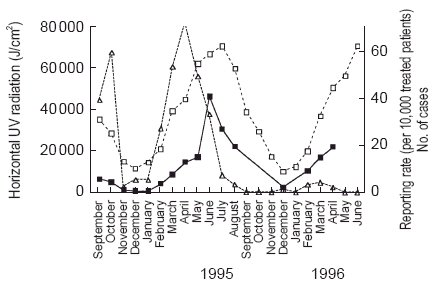
Monthly number of cases (triangles, right vertical axis), reporting rate (per 10 000 treated patients) for phototoxic reactions to sparfloxacin (full squares), and mean cumulative horizontal solar u.v. radiation in France (u.v. in J/cm2) (open squares). Restrictive regulatory measures were taken in June 1995.
Figure 2.
Relationship between u.v. radiation and the reporting rate for phototoxic reactions.
Discussion
The reactions observed during treatment with sparfloxacin are phototoxic reactions: the symptoms appear minutes to hours after sun exposure, and usually peak a few hours to several days later. Traditionally, the clinical signs and symptoms of phototoxicity are similar to the sunburn responses induced by u.v.B. In the case of sparfloxacin, there seems to exist a potentiation between u.v.A and u.v.B radiation [7].
Phototoxicity is a known effect of fluoroquinolones, and most fluoroquinolones seem able to cause phototoxicity (and perhaps photocarcinogenesis to a greater or lesser degree) [4, 8–11]: in the UK, a postmarketing study of oral ciprofloxacin demonstrated phototoxicity in 2 of 37 000 treated patients (5.4/100 000) [12]. The risk of phototoxicity associated with different fluoroquinolones seems different, the risk being higher with lomefloxacin and sparfloxacin than with others [10, 13]. Post-marketing surveillance in the US led to an estimated risk of phototoxicity with lomefloxacin of 70 per 100 000 prescriptions [2], as result of which a warning was issued to prescribers. The rank order of reported or experimental phototoxicity with fluoroquinolones [14] is essentially the same as that found from spontaneous reporting rates in France.
Sparfloxacin was approved in Japan in July 1993. Phototoxicity had been reported in premarketing clinical trials with an incidence of 0.4%, and a warning was thus included in the Summary of Product Characteristics. Despite this warning, 53 cases of phototoxicity were reported (incidence 0.53%) in postmarketing studies during the first year of marketing (Japanese Ministry of Health and Welfare, personal communication).
The purpose of this study was partly to confirm the occurrence of a higher rate of phototoxic reactions with sparfloxacin than with other fluoroquinolones, which has been demonstrated in vitro and in smaller patient series. However, we have also tried to confirm the influence of u.v. radiation (observed in vitro), not only on individual patients but at the population level during real drug utilization. The comparison of reporting rates and solar irradiation provides a near-experimental confirmation of the direct u.v.-dependent phototoxic effect of the drug, and confirms the role of natural sunlight (u.v.A + u.v.B) in the occurrence of these phototoxic reactions. The strength of the relationship provides indirect evidence for the suggested photoaugmentation by u.v.A and u.v.B [7]. This is also the first study where the relationship between u.v. energy and the occurrence of adverse drug reactions has been reported on a nationwide basis. Indeed, this appears to be the first reported epidemiological demonstration of an environmental influence on an adverse reaction.
The second aspect of our study relates to the effects of regulatory measures. The effect of regulatory restriction on the reporting rates of an adverse drug reaction are rarely reported, where the difference between the public health risk and individual patient risk is clearly shown.
After severe restrictions were placed on the use of sparfloxacin, the number of reported cases decreased drastically, in effect reducing the public health risk. However, this related to the decrease in the number of users and not to the decrease in individual user risk. When the number of reports to sales were related, the yearly reporting rate remained remarkably stable at 44 per 100 000 patients treated (assuming the same mean dose and duration of treatment). Individual patients prescribed sparfloxacin thus seem to be exposed to exactly the same phototoxic risk, despite the warnings and restrictions stated in the SPC, and the existence of a highly specific, well documented risk factor. There could be several explanations for this (a) the risk is unavoidable because exposure to sunlight during treatment is unavoidable; (b) avoidable risk factor was not emphasized by the physician and/or neglected by the patients; and (c) the real reduction in individual risk may in fact have been masked by the increased reporting of events due to greater physician awareness. It should be noted (Figure 1) that there was a peak in the reporting rate in June 1995, when the restrictive measures were announced.
In conclusion, beyond confirming the known phototoxic risk of sparfloxacin, this study also demonstrates for the first time the linear relationship between an adverse drug reaction and an environmental factor. After restrictive regulatory measures and warnings were introduced, there was a marked decrease in the number of reports, although the actual reporting rate of phototoxicity with sparfloxacin did not change.
References
- 1.Zelickson A. Phototoxic reaction with nalidixic acid. JAMA. 1964;190:556. doi: 10.1001/jama.1964.03070190076025. [DOI] [PubMed] [Google Scholar]
- 2.Ball P, Tillotson G. Tolerability of fluoroquinolone antibiotics. Past, present and future. Drug Safety. 1995;13:343–358. doi: 10.2165/00002018-199513060-00004. [DOI] [PubMed] [Google Scholar]
- 3.Ferguson J, Johnson BE. Ciprofloxacin-induced photosensitivity: in vitro and in vivo studies. Br J Dermatol. 1990;123:9–20. doi: 10.1111/j.1365-2133.1990.tb01819.x. [DOI] [PubMed] [Google Scholar]
- 4.Ferguson J, Johnson BE. Clinical and laboratory studies of the photosensitizing potential of norfloxacin, a 4-quinolone broad-spectrum antibiotic. Br J Dermatol. 1993;128:285–295. doi: 10.1111/j.1365-2133.1993.tb00173.x. [DOI] [PubMed] [Google Scholar]
- 5.Moore N, Biour M, Paux G, et al. Adverse drug reaction monitoring: doing it the French way. Lancet. 1985;ii:1056–1058. doi: 10.1016/s0140-6736(85)90918-3. [DOI] [PubMed] [Google Scholar]
- 6.Royer RJ. Adverse drug reactions with fluoroquinolones. Therapie. 1996;51:414–416. [PubMed] [Google Scholar]
- 7.Tokura Y, Iwamoto Y, Mizutani K, Takigawa M. Sparfloxacin phototoxicity: potential photoaugmentation by ultraviolet A and B sources. Arch Dermatol Res. 1996;288:45–50. doi: 10.1007/BF02505042. [DOI] [PubMed] [Google Scholar]
- 8.Ferguson J, Dawe R. Phototoxicity in quinolones: comparison of ciprofloxacin and grepafloxacin. J Antimicrob Chemother. 1997;40(Suppl A):93–98. doi: 10.1093/jac/40.suppl_1.93. [DOI] [PubMed] [Google Scholar]
- 9.Martinez LJ, Sik RH, Chignell CF. Fluoroquinolone antimicrobials: singlet oxygen, superoxide and phototoxicity. Photochem Photobiol. 1998;67:399–403. [PubMed] [Google Scholar]
- 10.Lipsky BA, Baker CA. Fluoroquinolone toxicity profiles: a review focusing on newer agents. Clin Infect Dis. 1999;28:352–364. doi: 10.1086/515104. [DOI] [PubMed] [Google Scholar]
- 11.Scheife RT, Cramer WR, Decker EL. Photosensitizing potential of ofloxacin. Int J Dermatol. 1993;32:413–416. doi: 10.1111/j.1365-4362.1993.tb02810.x. [DOI] [PubMed] [Google Scholar]
- 12.Jick SS, Jick H, Dean AD. A follow-up safety study of ciprofloxacin users. Pharmacotherapy. 1993;13:461–464. [PubMed] [Google Scholar]
- 13.Owen K. Comparative grepafloxacin phototoxicity in mouse skin. J Antimicrob Chemother. 1998;42:261–264. doi: 10.1093/jac/42.2.261. [DOI] [PubMed] [Google Scholar]
- 14.Snyder RD, Cooper CS. Photogenotoxicity of fluoroquinolones in Chinese hamster V79 cells: dependency on active topoisomerase II. Photochem Photobiol. 1999;69:288–293. doi: 10.1562/0031-8655(1999)069<0288:pofich>2.3.co;2. [DOI] [PubMed] [Google Scholar]



