Abstract
Aims
To determine whether multiple doses of ziprasidone alter the steady-state pharmacokinetics of the component steroids, ethinyloestradiol and levonorgestrel, of an oral contraceptive; to evaluate the tolerability of a co-administered combined oral contraceptive and ziprasidone; and to compare plasma concentrations of prolactin in subjects taking a combined oral contraceptive with placebo or ziprasidone.
Methods
Nineteen women taking a combined oral contraceptive (ethinyloestradiol 30 µg day−1 and levonorgestrel 150 µg day−1) were enrolled into a double-blind, placebo-controlled, two-way crossover study. They received ziprasidone 40 mg day−1 in two divided daily doses or placebo for 8 days (days 8–15) in one of two 21 day treatment periods separated by a 7 day washout period. Venous blood samples were collected immediately before and up to 24 h after the morning dose of oral contraceptive and ziprasidone or placebo on day 15 of each 21 day treatment period. These were assayed for ethinyloestradiol and levonorgestrel and the resulting data used to derive pharmacokinetic data for these steroids. Additional samples were collected immediately before and 4 h after the morning dose of oral contraceptive and ziprasidone or placebo on day 15 of each 21 day treatment period for prolactin assay. All observed and volunteered adverse events were recorded throughout the study.
Results
The mean AUC(0,24 h), Cmax and tmax for ethinyloestradiol and the mean AUC(0,24 h) and Cmax for levonorgestrel during ziprasidone co-administration were not statistically significantly different from corresponding values occurring during placebo co-administration. The tmax for levonorgestrel was approximately 0.5 h longer. Concomitant therapy with a combined oral contraceptive and ziprasidone did not result in adverse events not previously seen with either preparation alone.
Conclusions
The findings of this study suggest that, based on pharmacokinetic and tolerability data, ziprasidone may be co-administered with ethinyloestradiol and levonorgestrel without loss of contraceptive efficacy or increased risk of adverse events.
Keywords: ziprasidone, contraceptive, ethinyloestradiol, interaction, levonorgestrel, pharmacokinetics
Introduction
Schizophrenia occurs in both men and women, and despite gender differences in the epidemiology of the condition, the total lifetime risk of schizophrenia until the age of 59 years appears to be almost the same for women as for men [1–3]. Although many studies suggest that women with schizophrenia have a better prognosis than men with the condition, the findings of the only epidemiologically based, long-term follow-up study of individuals with schizophrenia conducted to date, challenge this assumption [1, 4].
Although the majority of young adults with schizophrenia are men, the number of young women with the condition is appreciable. Many individuals with schizophrenia are therefore women of child-bearing potential, a substantial proportion of whom take an oral contraceptive. Furthermore, it has been proposed that the differences in the clinical course of schizophrenia might be accounted for by a protective effect of oestrogens [5], and oestrogens have been shown to be implicated in the regulation of mood and memory, to attenuate the sensitivity of D2 receptors in experimental animals and to influence the expression of genes coding for 5-HT receptors [1, 2, 6]. Clearly, there is potential for interaction between oestrogens and newer antipsychotic drugs, such as ziprasidone.
The most popular oral contraceptives are those containing both an oestrogen and a progesterone. Although there have been no reported interactions between these combined oral contraceptive preparations and dopamine receptor antagonists, loss of contraceptive efficacy had been attributed to interactions with other centrally active drugs, such as the barbiturates and hydantoins [7]. This loss of contraceptive efficacy appears to result from hepatic enzyme induction and/or increased plasma protein synthesis and binding, and consequential decreases in the free plasma concentrations of these steroids. The oestrogen component of most combined oral contraceptive preparations, ethinyloestradiol, is both metabolized by and induces hepatic cytochrome (CYP) 3A4 [8, 9]. Studies performed in vitro using human liver microsomes have shown that ziprasidone is also metabolized by CYP3A4, but does not significantly inhibit this, or any of the other five major CYP isoenzymes (CYP2D6, CYP2C9, CYP1A2, CYP2E1, CYP2C19), at clinically relevant concentrations [10].
Conventional antipsychotic drugs increase plasma prolactin levels because they block the inhibitory effect of dopamine on prolactin release from the pituitary. This is a serious drawback of these agents because elevated plasma prolactin levels can cause adverse events, such as amenorrhoea and galactorrhoea [11].
Here we report the findings of a study designed to determine if multiple doses of ziprasidone alter the steady-state pharmacokinetics of the component steroids, ethinyloestradiol and levonorgestrel, of an oral contraceptive; to evaluate the tolerability of a co-administered combined oral contraceptive and ziprasidone; and to compare plasma concentrations of prolactin in subjects taking a combined oral contraceptive with placebo or ziprasidone.
Methods
Subjects
The subjects were to be healthy premenopausal women within the age range of 18–55 years. Subjects were to weigh between 50 and 80 kg and be within 20% of their ideal weight for age, height, gender and frame [12]. None of the subjects had received any drug therapy (except contraceptive medication and paracetamol) in the 2 weeks preceding entry into the study, and none had received any investigational drug in the 4 weeks preceding entry into the study or during the study. The women had been using a combined oral contraceptive containing 30 µg ethinyloestradiol and 150 µg levonorgestrel (either Microgynon®[Schering] or Ovranette®[Wyeth]) for at least three menstrual cycles. None was pregnant or lactating and all provided written informed consent. Demographic characteristics are presented in Table 1.
Table 1.
Demographic characteristics.
| Characteristic | Total (n = 19) |
|---|---|
| Age (years) | |
| Mean | 28.1 |
| Range | 22–39 |
| Weight (kg) | |
| Mean | 66.3 |
| Range | 52–82 |
| Ethnic origin | |
| White | 19 |
The subjects were asked to complete a medical history questionnaire during screening and any subject considered by the investigator to have clinically significant disease was excluded. In addition, the subjects were required to have, at screening, routine haematology, clinical chemistry and urinalysis test results within the reference range, and be negative for hepatitis B surface and core antigens. Subjects with evidence of drug abuse, who drank more than 14 units of alcohol per week, or who smoked more than 10 cigarettes per week were excluded.
Protocol
This was a double-blind, placebo-controlled, two-way crossover study designed to investigate the effect of multiple doses of ziprasidone on: the steady-state pharmacokinetics of ethinyloestradiol and levonorgestrel in healthy women taking a combined oral contraceptive; plasma concentrations of prolactin; and the safety and tolerability of a co-administered combined oral contraceptive and ziprasidone. The study protocol was approved by the Ethics Committee of the study sites.
The study comprised two 21 day periods separated by intervals of at least 7 days. Each 21 day period coincided with the time during which the subjects were scheduled to take their oral contraceptive and each 7 day washout period coincided with the time during which the subjects were scheduled not to be taking their oral contraceptive. The subjects took one combined oral contraceptive tablet, containing ethinyloestradiol 30 µg and levonorgestrel 150 µg (either Microgynon®[Schering] or Ovranette®[Wyeth]), every day of each 21 day period, between 08.00 and 09.00 h. On days 1–7 of each 21 day period the subjects took their oral contraceptive without any other study medication. On days 8–15 of each 21 day period the subjects took either ziprasidone HCl 40 mg day−1 in two divided daily doses or matched placebo, according to a computer-generated randomization, together with their oral contraceptive tablet in the morning and between 20.00 and 21.00 h in the evening. The morning and evening doses of the study drugs were taken within 5 min of eating breakfast and dinner, respectively.
The study drugs were administered under supervision at the study centre on the mornings of days 1, 8–12, 16 and 19–21, and in the evenings of days 13–15. The remaining doses of the study drugs were taken by the subjects unsupervised at home. On day 1, the subjects were given diary cards on which to record the exact times at which they took their oral contraceptive.
Sampling and sample analysis
On day 15 of each 21 day period, 12 ml venous blood samples for the determination of plasma ethinyloestradiol and levonorgestrel concentrations were collected immediately before dosing and at intervals up to 24 h after the morning dose of oral contraceptive and ziprasidone or placebo. Additional 7 ml venous blood samples for the determination of plasma prolactin concentrations were collected immediately before dosing and 4 h after the morning dose of oral contraceptive and ziprasidone or placebo. All samples were collected in heparinized tubes and centrifuged at 4 °C for 10 min at 1500g within 1 h of collection. Aliquots of plasma were removed and stored at −20 °C until analysis.
Plasma concentrations of ethinyloestradiol were determined using a gas chromatography/mass spectrometry procedure that was previously developed and validated at CEPHAC Research Centre, Saint-Benoit, France. In the first step of this procedure ethinyloestradiol and the internal standard (2H4 ethinyloestradiol) were extracted from plasma by liquid/liquid extraction with ether/hexane (40/60 v/v). The ethinyloestradiol and internal standard were then derivatized with 3,5 bi-(trifluoromethyl) benzoyl chloride. The resulting derivatives were then selectively extracted and analysed by capillary column gas chromatography using detection by negative chemical ionization with methane as the reagent gas. As the mass spectrum of derivatized ethinyloestradiol shows an intense molecular ion at m/z 536 and that for the internal standard shows a molecular ion at m/z 540, selected ion monitoring at m/z 536 and m/z 540 were used to obtain the optimum ratio between sensitivity and selectivity (against endogenous substances in plasma). During validation, calibration in plasma for this assay was linear for ethinyloestradiol from 10 to 500 pg ml−1 and the limit of quantification was 10 pg ml−1. Intra-batch imprecision and inaccuracy, expressed as the percent coefficient of variation (and percentage bias), were 10.37 (0.30), 3.44 (−1.24), 2.79 (0.09) and 2.50 (−4.23) at theoretical concentrations of 10, 50, 200 and 500 pg ml−1, respectively. The precision and accuracy of the method at the limits of quantification were rechecked before analysing the experimental plasma samples and were found to be comparable with those obtained during the assay validation. The specificity of the method was demonstrated by checking the chromatograms obtained from six blank plasma samples spiked with 100 mg ml−1 ziprasidone; no chromatographic peak was detected at the retention times of ethinyloestradiol, 2-hydroxyethinyloestradiol, levonorgestrel and norethindrone.
Plasma concentrations of levonorgestrel were also determined using a gas chromatography/mass spectrometry procedure that was previously developed and validated at CEPHAC. In the first step of this procedure levonorgestrel and internal standard (norethindrone) were extracted from plasma by liquid/liquid extraction with ether/hexane (40/60 v/v). The levonorgestrel and internal standard were then isolated from the residue of the first extract using solid phase extraction with a C8 cartridge. After elution from the cartridge, the analyte and internal standard were reacted with pentafluorobenzyl hydroxylamine hydrochloride to form the syn- and antigeometric isomers of their respective oxime derivatives. These oxime derivatives of levonorgestrel and norethindrone were then selectively extracted and analysed by capillary column gas chromatography using detection by negative chemical ionization with methane as the reagent gas. As the mass spectrum of derivatized levonorgestrel shows an intense fragment at m/z 299 and the most intense fragment for the internal standard shows at m/z 285, selected ion monitoring at m/z 299 and m/z 285 were used to obtain the optimum ratio between sensitivity and selectivity (against endogenous substances in plasma). During validation, calibration in plasma for this assay was linear for levonorgestrel from 0.1 to 20 ng ml−1 and the limit of quantification was 0.1 ng ml−1. Intra-batch imprecision and inaccuracy, expressed as the percent coefficient of variation (and percentage bias), were 10.0 (0.00), 3.85 (4.00), 1.77 (13.00) and 3.85 (13.00) at theoretical concentrations of 0.1, 0.5, 5 and 20 ng ml−1, respectively. The precision and accuracy of the assay at the limits of quantification were rechecked before analysing the experimental plasma samples and were found to be comparable with the precision and accuracy obtained during the assay validation. The specificity of the method was demonstrated by checking the chromatograms obtained from six blank plasma samples spiked with 100 mg ml−1 ziprasidone; no chromatographic peak was detected at the retention times of ethinyloestradiol, 2-hydroxyethinyloestradiol, levonorgestrel and norethindrone.
The areas under the plasma concentration–time curves from time zero to 24 h (AUC(0, 24 h)) for ethinyloestradiol and levonorgestrel were estimated by the linear trapezoidal approximation using Siphar version 3.3 (Simed, Créteil, France). The maximum observed serum concentrations of ethinyloestradiol and levonorgestrel (Cmax), and the earliest times at which these occurred (tmax) were estimated directly from the experimental data.
Plasma concentrations of prolactin were determined using a validated radioimmunoassay test kit with standard ranging from 3 to 200 ng ml−1 (Medigenix Diagnostic reference PRL-IRMA). The normal mean (range) value specified for prolactin by this kit for premenopausal women was 8.6 ng ml−1 (2.7–19.7 ng ml−1) (n = 95).
Tolerability
All observed or volunteered treatment-emergent adverse events occurring during the study were recorded using the Coding Symbol Thesaurus of Adverse Reaction Terms (COSTART) dictionary. Routine haematology, clinical chemistry and urinalysis tests were performed at screening, on days 8, 13 and 16 of each 21 day period and on day 21 of the second 21 day period. Pregnancy tests were performed at screening, on day 16 of each 21 day period and 8 days after the end of the second 21 day period. Urine alcohol and drug screening tests were performed at screening, and on days 8 and 13 of each 21 day period. Physical examinations, vital sign assessments and 12-lead ECG were performed at screening, on days 8 and 16 of each 21 day period and on day 21 of the second 21 day period. The subjects recorded details of any intermenstrual bleeding, such as spotting and/or breakthrough bleeding, on diary cards provided on day 1 of the study.
Statistical analysis
It was estimated that a sample size of 16 completing subjects (eight per sequence group) would provide at least 80% power to detect a 20% difference in the AUC(0,24 h) and Cmax for ethinyloestradiol and levonorgestrel with a probability of 0.8 when testing at the 5% level.
Natural log-transformed AUC(0,24 h) and Cmax values, and untransformed tmax values were analysed in an analysis of variance (anova) model containing sequence, period and treatment effects (PROC GLM of SAS®). The sequence effect was tested using the subject within sequence mean square as the error term and the period effect was tested using the within subject mean square as the error term. Both sequence and period effects were tested for significance at the 5% level.
Geometric means and standard deviations were calculated for AUC(0,24 h) and Cmax. Arithmetic means and standard deviations were calculated for tmax. Adjusted means, and their variances and covariances, were then calculated using the least-squares means statement from SAS®. These were then used to estimate the adjusted difference between the treatment effects, their standard errors and 95% confidence intervals of the differences. For AUC(0,24 h) and Cmax, the antilog of the difference between the treatment effects and their 95% intervals were calculated.
Results
Subjects
Nineteen white premenopausal women aged 22–39 years entered the study (Table 1). All 19 completed treatment with oral contraceptive plus placebo and 18 completed treatment with oral contraceptive plus ziprasidone. One subject discontinued the study after experiencing adverse events and only completed the oral contraceptive plus placebo phase. The 18 subjects who completed both phases of the trial were evaluated for pharmacokinetics. However, two subjects were not included in the statistical analysis of AUC(0,24 h) because plasma levels of ethinyloestradiol and levonorgestrel were undetectable at 24 h and, thus, no AUC(0,24 h) was calculated for these subjects. All subjects were evaluated for safety.
Pharmacokinetics
The mean plasma ethinyloestradiol and levonorgestrel concentration–time curves during ziprasidone treatment and during placebo treatment are shown in Figures 1 and 2. The mean plasma ethinyloestradiol concentration–time curves during ziprasidone treatment and during placebo treatment were similar and demonstrated a biphasic decline after maximum concentrations were achieved. The mean plasma levonorgestrel concentration–time curves during ziprasidone treatment and during placebo treatment were also similar and also demonstrated a biphasic decline after maximum concentrations were achieved.
Figure 1.
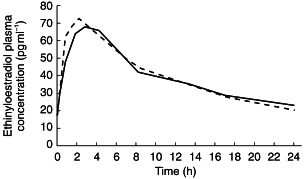
Mean plasma ethinyloestradiol concentration–time curves on day 15 during co-administration with ziprasidone (––) or placebo (––).
Figure 2.
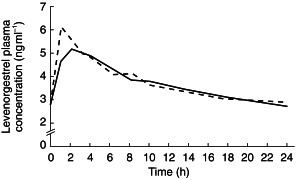
Mean plasma levonorgestrel concentration–time curves on day 15 during co-administration with ziprasidone (––) or placebo (––).
The pharmacokinetics of the component steroids of the oral contraceptive during treatment with ziprasidone were similar to those during treatment with placebo (Table 2). Comparisons of the AUC(0,24 h), tmax and Cmax for ethinyloestradiol showed no statistically significant differences between the ziprasidone and placebo treatments. Similarly, comparisons of the AUC(0,24 h), tmax and Cmax for levonorgestrel showed no statistically significant differences between the ziprasidone and placebo treatments, except for the tmax (P =0.03). However, the tmax for levonorgestrel during ziprasidone treatment was only approximately 0.5 h longer than that during placebo treatment.
Table 2.
Summary of pharmacokinetic parameters of ethinyloestradiol and levonorgestrel.
| Pharmacokinetic parameter | Oral contraceptive with placebo (n =18) | Oral contraceptive with ziprasidone (n =18) | Ratio (AUC and Cmax) or difference (tmax) | 95% confidence limits |
|---|---|---|---|---|
| Ethinyloestradiol | ||||
| AUC(0,24 h)a, c (pg ml−1 h) | 971 ± 225 | 964 ± 204 | 99.3% | 93.9,105.1 |
| Cmaxa (pg ml−1) | 77.1 ± 25.6 | 72.4 ± 22.9 | 93.9% | 85.6,103.0 |
| tmaxb (h) | 2.3 ± 1.2 | 2.9 ± 1.3 | 0.6 | −0.2,1.3 |
| Levonorgestrel | ||||
| AUC(0,24 h)a, c (ng ml−1 h) | 87.7 ± 23.7 | 85.7 ± 25.3 | 97.8% | 92.8,103.0 |
| Cmaxa (ng ml−1) | 6.42 ± 2.00 | 5.95 ± 1.86 | 92.7% | 84.5,101.8 |
| tmaxb (h) | 1.7 ± 1.0 | 2.3 ± 1.1 | 0.6 | 0.1,1.3 |
Geometric means and s.d. for AUC(0,24 h) and Cmax.
Arithmetic means and s.d. for tmax.
Excludes two subjects. One of these subjects had plasma ethinyloestradiol concentrations below the limit of detection 24 h after taking ziprasidone and one had plasma ethinyloestradiol concentrations below the limit of detection at 18 and 24 h after taking ziprasidone.
In the analyses, statistically significant sequence effects were observed for the ethinyloestradiol Cmax (P =0.008) and for the levonorgestrel AUC(0,24 h) (P =0.043), but these did not affect the interpretation of the results. There were no other sequence effects or interactions in the analysis of ethinyloestradiol or levonorgestrel.
Prolactin concentrations
The mean plasma prolactin concentrations, measured on day 15 (the eighth day of co-administration of oral contraceptive and ziprasidone or placebo), were higher during ziprasidone treatment than during placebo treatment, both immediately before the morning dose of study medication and 4 h after that dose (Table 3). However, the difference between groups in mean prolactin levels did not exceed 16 ng ml−1 and the change in pre and postdose levels within both groups was similar. Furthermore, while plasma prolactin concentrations during co-administration of oral contraceptive and ziprasidone, immediately before the morning dose of study medication, were above the normal range in 10 of 18 (56%) subjects, in 8 of these 10 subjects plasma prolactin concentrations had returned to within the normal range 4 h after dosing. Thus, plasma prolactin concentrations were above the normal range at both timepoints in only 2 (11%) subjects.
Table 3.
Mean plasma prolactin concentrations (ng ml−1) on day 15.
| Oral contraceptive with placebo (n =10) | Oral contraceptive with ziprasidone (n =18) | |||
|---|---|---|---|---|
| Time postdosing (h) | Meana | s.d. | Meana | s.d. |
| 0 | 8.80 | 4.79 | 22.57 | 14.33 |
| 4 | 3.09 | 1.47 | 18.96 | 8.65 |
A value of 1.5 ng ml −1 was substituted for individual values that were below the limit of detection.
Tolerability
Treatment-emergent adverse events were observed in, or volunteered by all 19 subjects at some time during the study. However, a substantial proportion of these subjects experienced treatment-emergent adverse events during the oral contraceptive plus placebo phase and some experienced them while receiving oral contraceptive alone (Table 4). The majority of adverse events were of mild-to-moderate severity and of short duration. The only severe adverse events were one case of agitation and one of tremor, both occurring in subjects taking both the oral contraceptive and ziprasidone. None was considered serious. Mild menstrual disturbances were reported by two subjects – one taking the oral contraceptive plus placebo and one taking oral contraceptive plus ziprasidone.
Table 4.
Treatment-emergent adverse events
| Adverse eventa | Oral contraceptive (n =19) | Oral contraceptive plus placebo (n =19) | Oral contraceptive plus ziprasidone (n =19) |
|---|---|---|---|
| Subjects with adverse events | 5 | 11 | 18 |
| Adverse events | 5 | 16 | 47 |
| Subjects discontinued due to adverse events | 0 | 0 | 1 |
| Asthenia | 0 | 1 | 13 |
| Somnolence | 0 | 0 | 6 |
| Headache | 3 | 6 | 3 |
| Nausea | 0 | 0 | 3 |
| Agitation | 0 | 0 | 3 |
| Insomnia | 0 | 0 | 3 |
| Myalgia | 0 | 2 | 2 |
| Abnormal dreams | 0 | 0 | 2 |
| Otherb | 2 | 7 | 12 |
Subjects with multiple occurrences of the same adverse event were counted once only.
Includes all other treatment-emergent adverse events, none of which occurred in more than one subject in any treatment group.
The subject who discontinued the study after completing the oral contraceptive plus placebo phase experienced multiple mild adverse events of moderate severity – dizziness, nausea, asthenia (tiredness) and vomiting – on the first day of treatment with oral contraceptive plus ziprasidone. All of these adverse events resolved before the subject discontinued study treatment on the next day.
Clinically significant laboratory test abnormalities occurred in three subjects during oral contraceptive plus ziprasidone treatment. One subject’s haematocrit decreased by more than 20% from 41.9% at screening to 33.3% by the end of the oral contraceptive plus ziprasidone phase. Most of this decrease occurred before the start of ziprasidone. Another subject tested positive in qualitative tests for urine glucose during the oral contraceptive plus ziprasidone phase, but negative at the end of ziprasidone treatment. A third subject experienced a decrease in serum calcium concentrations to 2.14–2.17 mmol l−1 during oral contraceptive plus placebo treatment, and a decrease to 2.11 mmol l−1 by the end of ziprasidone treatment. None of these laboratory test abnormalities necessitated discontinuation from the study.
Discussion
Results from this study indicate that multiple doses of ziprasidone do not have a clinically significant effect on the pharmacokinetics of the component steroids of a combined oral contraceptive. Co-administration of ziprasidone did not influence the pharmacokinetics of ethinyloestradiol, as indicated by the AUC(0,24 h), Cmax and tmax, and did not influence the pharmacokinetics of levonorgestrel as indicated by the AUC(0,24 h) and Cmax. Although co-administration of ziprasidone was associated with an increase in the tmax for levonorgestrel, this increase was only approximately 0.5 h and is not clinically significant as it was substantially less than both the dosing interval (12 h) and the variation in tmax between individuals.
The lack of a clinically significant pharmacokinetic interaction between ziprasidone and this combined oral contraceptive indicates that, although ziprasidone is a CYP3A4 substrate, it does not inhibit or induce this isoenzyme sufficiently to alter the pharmacokinetics of ethinyloestradiol, which is also a CYP3A4 substrate [9, 10]. The results also suggest that ziprasidone does not affect sex-hormone-binding globulin. Based on these data we can therefore conclude that ziprasidone will not influence the contraceptive efficacy, tolerability or other effects of the combined oral contraceptive pill.
Findings from this study also indicate that ziprasidone is associated with a modest elevation in plasma prolactin concentrations in premenopausal women. However, the mean plasma prolactin concentration on day 15 was only 16 ng ml−1 greater in the ziprasidone group than in the placebo group. As such, the effect of ziprasidone on prolactin secretion is minimal compared with the effects of conventional antipsychotic drugs, such as haloperidol, and the newer antipsychotic agent, risperidone, which have been associated with marked persistent elevations in premenopausal women being treated for schizophrenia, and with clinical sequelae such as galactorrhoea and amenorrhoea [11, 13, 14]. In one study involving five premenopausal women, the mean plasma prolactin concentration during risperidone treatment was 124 ng ml−1, three women developed galactorrhoea and all five developed amenorrhoea [13]. In a larger, more recent study involving 20 premenopausal women, risperidone was associated with significantly higher prolactin levels than conventional antipsychotic agents (102 ng ml−1vs 48 ng ml−1, P<0.0001); two women developed galactorrhoea and six developed amenorrhoea during risperidone treatment [14].
The effect of ziprasidone on plasma prolactin concentration in women observed in this study may differ slightly from the effect observed in men, in whom ziprasidone has been associated with only marginal, transient elevations that return to baseline within the dosing interval [15]. This is to be expected, as elevations in plasma prolactin concentrations in women receiving conventional antipsychotic drugs are greater than in men [16–18]. This effect may be attributable to hormonal influences such as oestrogen-mediated enhancement of prolactin releasing stimuli and the number of lactotrophic cells of the anterior pituitary [18]. Such hormonal influences may also be relevant to the effects of ziprasidone on prolactin secretion in women. The exact magnitude of any such effect is difficult to discern from the findings of the present study, however, because inter-individual variability in prolactin concentrations was large. Nevertheless, the effect would appear to be small as plasma prolactin concentrations persistently above the normal range were only rarely observed.
The finding that ziprasidone is associated with only a relatively modest increase in prolactin is encouraging as this predicts that adverse effects attributable to marked and persistent elevations associated with other antipsychotic agents are less likely to be encountered. It has been suggested that women with hyperprolactinaemia may be at greater risk of developing osteoporosis, androgen excess and hirsutism, in addition to better-known adverse effects such as endometrial hyperplasia, amenorrhoea and galactorrhoea [11].
In addition to evaluating the pharmacokinetics of the component steroids of a combined oral contraceptive and plasma prolactin levels, this study also evaluated the safety and tolerability of a co-administered combined oral contraceptive and ziprasidone. There were no serious adverse events and most of the mild-to-moderate adverse events that did occur were predictable on the basis of the receptor binding profile of ziprasidone and were similar to those previously seen with ziprasidone monotherapy [15, 19, 20]. Although one subject experienced multiple adverse events while receiving oral contraceptive plus ziprasidone and withdrew from the study, all of these adverse events resolved before treatment was discontinued the next day. Only two subjects experienced menstrual disturbances. One subject had irregular menstruation during placebo treatment and the other had a shortened menstruation during ziprasidone treatment.
In conclusion, the findings of this study indicate that the co-administration of multiple doses of ziprasidone and a combined oral contraceptive does not have a clinically significant effect on the pharmacokinetics of ethinyloestradiol or levonorgestrel. They also suggest that this combination of oral contraceptive and ziprasidone does not produce any clinically significant adverse effects or produce marked elevations of plasma prolactin concentrations.
References
- 1.Kulkarni J. Women and schizophrenia: a review. Austr NZ J Psychiatry. 1997;31:46–56. doi: 10.3109/00048679709073798. [DOI] [PubMed] [Google Scholar]
- 2.Häfner H, van der Heiden W. Epidemiology of schizophrenia. Can J Psychiatry. 1997;42:139–151. doi: 10.1177/070674379704200204. [DOI] [PubMed] [Google Scholar]
- 3.Jablensky A, Sartorius N, Ernberg G, et al. Schizophrenia: manifestations, incidence and course in different cultures. A World Health Organization ten-country study. Psychol Med Monogr. 1992;20(Suppl):1–97. doi: 10.1017/s0264180100000904. [DOI] [PubMed] [Google Scholar]
- 4.Kendler KS, Walsh D. Gender and schizophrenia: results of an epidemiologically based family study. Br J Psychiatry. 1995;167:184–192. doi: 10.1192/bjp.167.2.184. [DOI] [PubMed] [Google Scholar]
- 5.Seeman MV, Lang M. The role of oestrogens in schizophrenia: gender differences. Schizophr Bull. 1990;16:185–195. doi: 10.1093/schbul/16.2.185. [DOI] [PubMed] [Google Scholar]
- 6.Fink G, Sumner BE, Rosie R, et al. Estrogen control of central mood, mental state and memory. Cell Mol Neurobiol. 1996;16:325–344. doi: 10.1007/BF02088099. [DOI] [PubMed] [Google Scholar]
- 7.Banck DJ, Orme MLE. Pharmacokinetic drug interactions with oral contraceptives. Clin Pharmacokin. 1990;18:472–484. doi: 10.2165/00003088-199018060-00004. [DOI] [PubMed] [Google Scholar]
- 8.Pichard L, Fabre G, Fabre J, et al. Cyclosporin A drug interactions: screening for inducers and inhibitors of cytochrome P450 (cyclosporin A oxidase) in primary human hepatocytes and liver microsomes. Drug Metab Dispos. 1990;18:595–606. [PubMed] [Google Scholar]
- 9.Smith DA. Species differences in metabolism and pharmacokinetics: are we close to understanding? Drug Metab Rev. 1991;23:355–373. doi: 10.3109/03602539109029764. [DOI] [PubMed] [Google Scholar]
- 10.Prakash C, Kamel A, Cui D, et al. Identification of the major human liver cytochrome P450 isoform responsible for the primary metabolites of ziprasidone and prediction of possible drug interactions. Br J Clin Pharmacol. 2000;49(Suppl. 1):35S–42S. doi: 10.1046/j.1365-2125.2000.00151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ataya K, Mercado A, Kartaginer J, et al. Bone density and reproductive hormones in patients with neuroleptic-induced hyperprolactinemia. Fertil Steril. 1988;50:876–881. doi: 10.1016/s0015-0282(16)60365-5. [DOI] [PubMed] [Google Scholar]
- 12.Metropolitan height and weight tables Stat Bull Metrop Life Found. 1983;64:3–9. [PubMed] [Google Scholar]
- 13.Dickson RA, Dalby JT, Williams R, et al. Risperidon- induced prolactin elevations in premenopausal women with schizophrenia. Am J Psychiatry. 1995;152:1102–1103. doi: 10.1176/ajp.152.7.1102b. [DOI] [PubMed] [Google Scholar]
- 14.Ananthamoorthy R, Caracci G. Prolactin levels of premenopausal women treated with risperidone and conventional neuroleptics. Presented at the 150th Annual Meeting of the American Psychiatric Association (APA), San Diego, USA, 17–22 May, 1997, NR 164.
- 15.Miceli JJ, Wilner KD, Hansen RA, et al. Single- and multiple-dose pharmacokinetics of ziprasidone under non-fasting conditions in healthy male volunteers. Br J Clin Pharmacol. 2000;49(Suppl. 1):5S–13S. doi: 10.1046/j.1365-2125.2000.00147.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.King DJ, Devaney N, Cooper SJ. Pharmacokinetics and antipsychotic effect of remoxipride in chronic schizophrenic patients. J Psychopharmacol. 1990;2:83–89. doi: 10.1177/026988119000400206. [DOI] [PubMed] [Google Scholar]
- 17.Lahdelma RL, Appelberg B, Kuoppasalmi K, et al. Plasma concentrations of remoxipride and haloperidol in relation to prolactin and short-term therapeutic outcome in schizophrenic patients. Eur Neuropsychopharmacol. 1991;1:535–540. doi: 10.1016/0924-977x(91)90007-h. [DOI] [PubMed] [Google Scholar]
- 18.Kuruvilla A, Peedicayil J, Srikrishna G, et al. A study of serum prolactin levels in schizophrenia: comparison of males and females. Clin Exp Pharmacol Physiol. 1992;19:603–606. doi: 10.1111/j.1440-1681.1992.tb00511.x. [DOI] [PubMed] [Google Scholar]
- 19.Seeger TF, Seymour AW, Schmidt AW, et al. Ziprasidone (CP-88,059): a new antipsychotic with combined dopamine and serotonin receptor antagonist activity. J Pharmacol Exp Ther. 1995;275:101–113. [PubMed] [Google Scholar]
- 20.Wilner KD, Tensfeldt TG, Baris B, et al. Single- and multiple-dose pharmacokinetics of ziprasidone in healthy young and elderly volunteers. Br J Clin Pharmacol. 2000;49(Suppl. 1):15S–20S. doi: 10.1046/j.1365-2125.2000.00148.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


