Abstract
Aims
To compare the pharmacokinetics of ziprasidone in healthy young (18–45 years) men and women, and healthy elderly (≥ 65 years) men and women.
Methods
Eight young men, 11 young women, 8 elderly men and 8 elderly women were given oral ziprasidone 40 mg day−1, in two evenly divided daily doses, for 7 days, followed by a single 20 mg dose on day 8. Serum samples were collected immediately before the morning dose on days 1–8, for up to 12 h after dosing on day 1 and for up to 96 h after dosing on day 8. The resulting data were used to derive pharmacokinetic parameters of ziprasidone in each age and gender group.
Results
Steady-state serum concentrations of ziprasidone were achieved within 2–3 days. The steady-state pharmacokinetics of ziprasidone, determined 8 days after the initiation of treatment, were similar in the young men, elderly men and young women. Assessment of gender effects by analysis of variance revealed statistically significant differences in Cmax (85 vs 69 ng ml−1) and tmax (3.19 vs 4.81 h) but no differences in AUC(0,12 h) or λz. Assessment of age effects by analysis of variance revealed statistically significant differences in AUC(0,12 h) (560 vs 465 ng ml−1h), Cmax (85 vs 69 ng ml−1) and λz (0.126 vs 0.197 l h−1) but no difference in tmax. Assessment of age and gender effects by analysis of covariance, with body weight as the covariate, did not reveal any significant differences. The mean t½,z in the young men, young women, elderly men and elderly women were 3.1, 4.1, 5.7 and 5.3 h, respectively. Standard deviations of the means for the pharmacokinetic parameters for the elderly women tended to be large.
Conclusions
The influence of age and gender on the pharmacokinetics of ziprasidone is not clinically significant.
Keywords: age, gender, pharmacokinetics, schizoaffective, schizophrenia, ziprasidone
Introduction
A previous study in healthy men has shown that ziprasidone has a pharmacokinetic profile which is characterized by rapid attainment of steady state and dose-proportional Cmax and AUC(0,12 h) [1]. Ziprasidone appears to be extensively metabolized and less than 1% is excreted unchanged in the urine or faeces. The major metabolites of ziprasidone are inactive [2].
Studies to characterize the pharmacokinetics of ziprasidone that have been undertaken to date have involved mainly young men. However, the target population for ziprasidone in clinical practice will include men and women of all ages. Here we report the findings of a study designed to evaluate the effects of age and gender on the pharmacokinetics of ziprasidone.
Methods
Subjects
The study subjects were healthy young (18–45 years) and elderly (≥ 65 years) men and women. All subjects had a body weight ≤ 91 kg. The young subjects were within 10% of the ideal weight for age and height [3] and the elderly subjects were within 20% of their ideal weight. The women had been surgically sterilized, were at least 2 years postmenopausal or had been practising successful contraception for at least 3 months. Pregnant or lactating women were excluded from the study.
The study protocol was approved by an independent institutional review board. All subjects provided written informed consent.
Study design
This was an open-label, multiple-dose study designed to evaluate the pharmacokinetics of ziprasidone at steady-state in healthy young men, young women, elderly men and elderly women.
Ziprasidone was administered orally at a dose of 40 mg day−1, in two evenly divided daily doses for 7 days, followed by a single dose on the morning of day 8. The morning dose of ziprasidone was given with 50 ml water immediately after a standard breakfast which was eaten over a 20 min period. The evening dose was given with 50 ml water immediately after a standard dinner which was also eaten over a 20 min period. The interval between the morning and evening dose was approximately 12 h.
Pharmacokinetic sampling
Blood samples for the determination of single-dose pharmacokinetic parameters were collected before dosing and for up to 12 h after the morning dose of ziprasidone on day 1. Samples for the determination of steady-state pharmacokinetic parameters were obtained immediately before dosing and up to 96 h after the morning dose of ziprasidone on day 8. Additional samples for the determination of trough serum levels of ziprasidone were collected immediately before the morning dose on days 2, 3, 4, 5, 6 and 7.
Plasma protein-binding (Fb) determinations were undertaken in samples taken immediately before administration of the last dose of ziprasidone on day 8.
Pharmacokinetic analysis
Serum concentrations of ziprasidone were determined using high-performance liquid chromatography (h.p.l.c.) involving solid-phase extraction and u.v. detection. The assay had a dynamic range of 1.0–250.0 ng ml−1[4]. Ziprasidone concentrations below the lower limit of quantification were assigned a value of 0 ng ml−1 in pharmacokinetic calculations.
The maximum observed serum concentration of ziprasidone (Cmax) and the earliest time at which Cmax occurred (tmax) were estimated directly from the experimental data. The area under the serum concentration-time curve to the 12th hour after dosing (AUC(0, 12 h)) was estimated by linear trapezoidal approximation, and the accumulation ratio was calculated as the AUC(0,12 h) on day 8 divided by AUC(0,12 h) on day 1.
The elimination rate constant (λz), was derived from least-squares regression analysis of the serum concentration-time data obtained during the terminal log-linear phase. The half-life (t½,z) was calculated as ln 2/λz and mean t½,z was calculated as ln 2/mean λz.
Statistical analysis
It was estimated that a sample size of eight completing subjects per group (i.e. young men, young women, elderly men and elderly women) would provide 80% power to detect at least a 75% between-group difference in the AUC on day 8, at a significance level of 5%.
Analysis of AUC(0,12 h) and Cmax was undertaken on natural log-transformed data; other variables were unadjusted. Each pharmacokinetic parameter was analysed as a dependent variable in a two-way analysis of variance model that used age, gender and the age-by-gender interaction as classification variables (PROC GLM of SAS®).
Differences in AUC(0,12 h) and Cmax were also tested by means of a two-way analysis of covariance model in which age, gender and the age-by-gender interaction were classification variables and body weight was a continuous covariate. Student’s t-tests with 95% confidence limits were used to examine pair-wise differences between the groups. Values of P< 0.05 were considered to be statistically significant.
Results
Subjects
Thirty-five subjects participated in the study: eight subjects each were young men, elderly men and elderly women, and 11 were young women.
The demographic characteristics of the subject groups are summarized in Table 1. The majority of the subjects were Caucasian. There was one Black young man and one Black and one Hispanic young woman.
Table 1.
Baseline demographic characteristics
| Young men (n = 8) | Young women (n =11) | Elderly men (n =8) | Elderly women (n =8) | |
|---|---|---|---|---|
| Mean age (range) (years) | 24 (18–39) | 31 (18–44) | 70 (66–74) | 70 (65–76) |
| Mean weight (range) (kg) | 74 (61–90) | 65 (44–84) | 79 (72–93) | 62 (50–73) |
| White | 7 | 9 | 8 | 8 |
| Black | 1 | 1 | 0 | 0 |
| Hispanic | 0 | 1 | 0 | 0 |
Three of the young women withdrew from the study within the first 3 days of treatment because of treatment-related adverse events (mild lack of energy; mild nausea and dizziness, and a road traffic accident). These subjects were excluded in the analysis of pharmacokinetic data.
Single-dose pharmacokinetics
Serum ziprasidone concentration-time curves generated following the administration of a single 20 mg dose of ziprasidone are shown in Figure 1. The mean AUC(0,12 h) on day 1 was approximately 11% greater in the elderly than in the young subjects (382 ng ml−1h vs 344 ng ml−1 h), and mean Cmax was approximately 7% greater in the elderly than in the young subjects (60 ng ml−1vs 56 ng ml−1; Table 2). Exposure to ziprasidone in the elderly women, measured by the AUC(0,12 h), was approximately 14% greater than in the elderly men (408 ng ml−1 h vs 358 ng ml−1 h), and the Cmax in the elderly women was approximately 22% greater than in the elderly men (66 ng ml−1vs 54 ng ml−1). The mean tmax after the first dose of ziprasidone was between 4 and 5 h in all subject groups.
Figure 1.
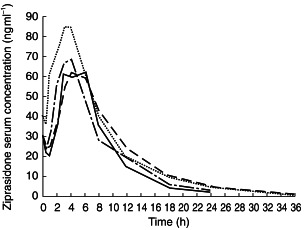
Mean ziprasidone serum concentrations following administration of a single ziprasidone 20 mg dose to healthy young men (–––), young women (-·-·), elderly men (----) and elderly women (…..).
Table 2.
Summary of pharmacokinetic parameters (mean ± s.d.) following single 20 mg dose of ziprasidone
| Men (n = 16) | Women (n = 16) | Men and women (n = 32) | |
|---|---|---|---|
| Young subjects | |||
| AUC(0,12 h)a (ng ml−1 h) | 359 ± 107 | 330 ± 61 | 344 ± 84 |
| AUC(0,12 h)a normalizedb (ng ml−1 h) | 343 ± 142 | 332 ± 87 | 337 ± 113 |
| Cmaxa (ng ml−1) | 60 ± 13 | 53 ± 16 | 56 ± 14 |
| Cmaxa normalizedb (ng ml−1) | 57 ± 19 | 53 ± 18 | 55 ± 18 |
| tmax (h) | 4 ± 1 | 4 ± 1 | 4 ± 1 |
| Elderly subjects | |||
| AUC(0,12 h)a (ng ml−1 h)b | 358 ± 51 | 408 ± 115 | 382 ± 86 |
| AUC(0,12 h)a normalizedb (ng ml−1 h) | 319 ± 69 | 463 ± 158 | 384 ± 129 |
| Cmaxa (ng ml−1) | 54 ± 8 | 66 ± 22 | 60 ± 16 |
| Cmaxa normalizedb (ng ml−1) | 48 ± 11 | 75 ± 27 | 60 ± 22 |
| tmax (h) | 4 ± 2 | 5 ± 1 | 5 ± 2 |
Geometric means and standard deviations, normalized.
Normalized to a body weight of 70 kg.
Steady-state pharmacokinetics
Serum ziprasidone concentration–time curves on day 8 are shown in Figure 2. Visual inspection of trough serum ziprasidone concentrations suggested that steady state was attained within 2–3 days of the start of dosing. The mean accumulation ratios, calculated as the ratio of AUC(0,12 h), day 8/AUC(0,12 h), day 1, were similar in all four groups. These were 1.27, 1.46, 1.43 and 1.54 in the young men, young women, elderly men and elderly women, respectively. Among the young and elderly of both genders the mean accumulation ratios were 1.36 and 1.48, respectively.
Figure 2.
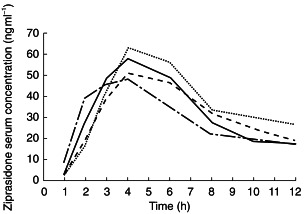
Mean ziprasidone serum concentrations on day 8 following administration of ziprasidone 20 mg twice daily for 8 days to healthy young men (–––), young women (-·-·), elderly men (----) and elderly women (…..).
Based on steady-state AUC(0,12 h), Cmax, tmax and λz values, the pharmacokinetics of ziprasidone in young men were similar to those in young women (Table 3). In addition, overall exposure based on AUC(0,12 h) and Cmax was similar among elderly men, young men and young women. However, exposure in elderly women was greater than that in elderly men (23% for AUC(0,12 h) and 44% for Cmax). Normalizing AUC(0,12 h) and Cmax values for body weight (70 kg) did not account for this difference in exposure (Table 3).
Table 3.
Summary of pharmacokinetic parameters (mean±s.d.) at steady state following administration of ziprasidone, 40 mg day−1 for 8 days.
| Young men (n = 8) | Young women (n = 8) | Young men and women (n = 16) | Elderly men (n = 8) | Elderly women (n = 8) | Elderly men and women (n = 16) | |
|---|---|---|---|---|---|---|
| AUC(0,12 h)a (ng ml−1 h) | 451 ± 145 | 479 ± 83 | 465 ± 117 | 505 ± 98 | 621 ± 159 | 560 ± 137 |
| AUC(0,12 h)a normalizedb (ng ml−1 h) | 431 ± 182 | 481 ± 133 | 455 ± 159 | 450 ± 92 | 706 ± 212 | 563 ± 192 |
| Cmaxa (ng ml−1) | 67 ± 15 | 72 ± 13 | 69 ± 14 | 71 ± 17 | 102 ± 40 | 85 ± 31 |
| Cmaxa normalizedb (ng ml−1) | 64 ± 22 | 72 ± 19 | 68 ± 20 | 63 ± 15 | 115 ± 50 | 85 ± 39 |
| tmax (h) | 4 ± 2 | 3 ± 1 | 4 ± 1 | 6 ± 2 | 3 ± 2 | 4 ± 2 |
| λz (l h−1) | 0.225 ± 0.059 | 0.169 ± 0.030 | 0.197 ± 0.0535 | 0.122 ± 0.057 | 0.130 ± 0.028 | 0.126 ± 0.043 |
| t½,zc (h) | 3.1 | 4.1 | 3.5 | 5.7 | 5.3 | 5.5 |
| Fb (%) | 99.87 ± 0.03 | 99.83 ± 0.11 | 99.85 ± 0.08 | 99.89 ± 0.02 | 99.86 ± 0.06 | 99.87 ± 0.04 |
Geometric means and standard deviations.
Normalized to a body weight of 70 kg.
Harmonic mean.
The unusual pharmacokinetics observed in the elderly women were principally due to two elderly women who had very high AUC(0,12 h) (867 and 881 ngml−1 h) and Cmax values (182 and 166 ng ml−1). These individuals can be identified in Figures 3 and 4.
Figure 3.
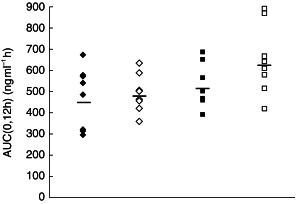
Individual AUC(0,12 h) following administration of ziprasidone 20 mg twice daily for 8 days to healthy young men (♦), young women (◊), elderly men (▪) and elderly women (□). Group means are denoted by horizontal lines.
Figure 4.
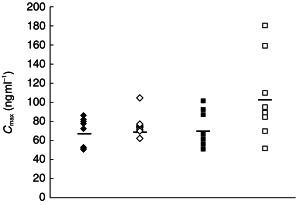
Individual Cmax following administration of ziprasidone 20 mg twice daily for 8 days to healthy young men (♦), young women (◊), elderly men (▪) and elderly women (□). Group means are denoted by horizontal lines.
In general, steady-state t½,z values were broadly similar in young men and young women (3.1 h vs 4.1 h; Table 3), and in elderly men and elderly women (5.7 h vs 5.3 h; Table 3). The mean t½,z in the elderly subjects (both genders) was 2 h longer than in the young subjects (both genders) (5.5 h vs 3.5 h).
The mean per cent plasma protein binding (Fb) was almost identical among the four groups (99.8% to 99.9%; Table 3).
Comparisons of AUC(0,12 h) and Cmax between the young and elderly subject groups by two-way analysis of variance showed small but statistically significantly greater ziprasidone exposure in elderly subjects (both genders) of between 20 and 23% (Table 4; P< 0.05). However, formal statistical analysis, performed using a two-way analysis of covariance model in which age, gender and the age-by-gender interactions were classification variables and body weight was a continuous covariate, revealed no significant differences between the AUC(0,12 h) and Cmax values for the young (both genders) and those for the elderly (both genders) (Table 4). The AUC(0,12 h) values for the elderly and the young differed by less than 20% (469 ng ml−1 h vs 555 ng ml−1 h; P > 0.05; Table 4). Similarly, the Cmax values for the elderly and the young differed by less than 20% (70 ng ml−1vs 84 ng ml−1; P > 0.05; Table 4).
Table 4.
Summary of steady-state pharmacokinetic parameters for ziprasidone according to age.
| Mean | Difference between means (95% CI) | |||
|---|---|---|---|---|
| Young | Elderly | P value | ||
| Analysis of variance | ||||
| AUC(0,12 h)a (ng ml−1 h) | 465 | 560 | 120% (100.9, 143.6) | 0.040 |
| Cmaxa (ng ml−1) | 69 | 85 | 122% (100.5, 148.9) | 0.045 |
| λz (h−1) | 0.197 | 0.126 | −0.071 (−0.104, −0.038) | 0.0001 |
| tmax (h) | 3.63 | 4.38 | 0.75 (−0.41, 1.91) | 0.194 |
| Analysis of covariance | ||||
| AUC(0,12 h)a (ng ml−1 h) | 469 | 555 | 118% (99.6, 140.4) | 0.056 |
| Cmaxa (ng ml−1) | 70 | 84 | 120% (99.1, 146.5) | 0.062 |
The difference between geometric means for AUC(0,12 h) and Cmax was represented by the ratio between geometric means and the 95% confidence interval (CI) around the ratio.
The analysis of covariance also revealed no significant differences between the AUC(0,12 h) and Cmax values for the men (both age groups) and those for the women (both age groups) (Table 5).The AUC(0,12 h) values for the men and the women differed by less than 5% (498 ng ml−1 h vs 522 ng ml−1 h; P > 0.05; Table 5). Similarly, the Cmax values for the men and the women differed by less than 20% (71 ng ml−1vs 82 ng ml−1; P > 0.05; Table 5).
Table 5.
Summary of steady-state pharmacokinetic parameters for ziprasidone according to gender.
| Mean | Difference between means (95% CI) | |||
|---|---|---|---|---|
| Women | Men | P value | ||
| Analysis of variance | ||||
| AUC(0,12 h)a (ng ml−1 h) | 545 | 477 | 114% (95.8, 136.2) | 0.134 |
| Cmaxa (ng ml−1) | 85 | 69 | 124% (102.0, 151.0); | 0.032 |
| λz (h−1) | 0.149 | 0.174 | −0.024 (−0.058, 0.009) | 0.142 |
| tmax (h) | 3.19 | 4.81 | −1.63 (−2.78, −0.47) | 0.008 |
| Analysis of covariance | ||||
| AUC(0,12 h)a (ng ml−1 h) | 522 | 498 | 105% (87.0, 126.1) | 0.635 |
| Cmaxa (ng ml−1) | 82 | 71 | 115% (93.4, 142.6) | 0.206 |
The difference between geometric means for AUC(0,12 h) and Cmax was represented by the ratio between geometric means and the 95% confidence interval (CI) around the ratio.
Discussion
The objective of this study was to evaluate the pharmacokinetics of oral ziprasidone at steady-state in individuals of different ages and genders.
All of the measured pharmacokinetic parameters varied by considerably less than 50% among the subject groups. Indeed, based on AUC(0,12 h), Cmax, tmax and λz values at steady state, there were no significant differences between the pharmacokinetics of ziprasidone in the young men and those in the young women. Overall exposure to ziprasidone, based upon AUC(0,12 h) and Cmax values in the elderly men was similar to that in the young men. Although exposure in the elderly women appeared to be greater than in the other groups, this relatively high exposure appeared to be attributable to two outlying individuals, who had both unusually high AUC(0,12 h) and Cmax values. There were no discernible reasons why these two elderly women had high AUC(0,12 h) and Cmax values. Neither had unusually low body weights and normalizing AUC(0,12 h) and Cmax values for body weight (70 kg) did not account for the small difference in exposure. It is possible therefore that the bioavailability of ziprasidone, administered orally with food, may be greater in some elderly women than in other individuals. However, the findings of this study do not demonstrate this conclusively.
Although the results showed that age and gender did not influence substantially the pharmacokinetics of ziprasidone, the relatively wide confidence intervals and small sample size do not preclude this possibility. However, there is no clinical evidence from patients with schizophrenia to suggest that there are any clinical differences between men and women and young and elderly patients treated with ziprasidone.
In a similar study, the pharmacokinetics of the antipsychotic agent, risperidone, were evaluated in eight healthy young subjects and 12 healthy elderly subjects. Following the administration of a single oral 1 mg dose, the AUC(0, ∞), tmax and t½,z values for the active moiety of risperidone, 9-hydroxy-risperidone, were significantly greater in the elderly subjects than in the young subjects. Thus, although the pharmacokinetics of risperidone itself in the elderly subjects were described as being comparable with those in the young subjects, the authors recommended careful dose titration of risperidone in the elderly [5].
In another study, involving 12 patients aged 63–85 years with chronic schizophrenia, the steady-state pharmacokinetics of the antipsychotic agent, quetiapine, were found to be influenced by age. Following the oral administration of quetiapine in stepwise incremental doses (25–250 mg per dose) the oral clearance of quetiapine was approximately 50% lower in these elderly patients compared with younger patients. The authors therefore stressed that the clinically effective dose of quetiapine for elderly patients may be 50% lower than that for younger patients [6].
In conclusion, the pharmacokinetics of ziprasidone do not appear to be influenced significantly by age or gender. These findings suggest that there is no need for a ziprasidone dosage adjustment for elderly subjects based on the pharmacokinetics of the drug.
References
- 1.Miceli JJ, Wilner KD, Hansen RA, et al. Single- and multiple-dose pharmacokinetics under non-fasting conditions in healthy male volunteers. Br J Clin Pharmacol. 2000;49(Suppl. 1):5S–13S. doi: 10.1046/j.1365-2125.2000.00147.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Howard HR, Lowe JA, Seeger TF, et al. 3 – Benzisothiazolylpiperazine derivatives as potential atypical antipsychotic agents. J Med Chem. 1996;39:143–148. doi: 10.1021/jm950625l. [DOI] [PubMed] [Google Scholar]
- 3.Metropolitan height and weight tables Stat Bull Metrop Life Found. 1983;64:3–9. [PubMed] [Google Scholar]
- 4.Janiszewski JS, Fouda HG, Cole RO. Development and validation of a high-sensitivity assay for an antipsychotic agent, CP-88,059, with solid-phase extraction and narrow-bore high-performance liquid chromatography. J Chromatogr. 1995;668:133–139. doi: 10.1016/0378-4347(95)00071-p. [DOI] [PubMed] [Google Scholar]
- 5.Snoeck E, Van Peer A, Sack M. Influence of age, renal and liver impairment on the pharmacokinetics or risperidone. Psychopharmacology. 1995;122:223–229. doi: 10.1007/BF02246543. [DOI] [PubMed] [Google Scholar]
- 6.Wong YWJ, Ewing BJ, Thrum PT. Multiple-dose pharmacokinetics of ‘Seroquel’ (quetiapine) in elderly psychotic patients. Schizophrenia Res. 1997;24:199–200. [Google Scholar]


