Abstract
Aims
To assess whether renal impairment influences the pharmacokinetics of ziprasidone, and to determine whether ziprasidone is cleared via haemodialysis.
Methods
Thirty-nine subjects with varying degrees of renal impairment were enrolled into an open-label, multicentre, multiple-dose study and assigned to four groups according to their renal function: normal (group 1, creatinine clearance > 70 ml min−1); mildly impaired (group 2, creatinine clearance 30–60 ml min−1); moderately impaired (group 3, creatinine clearance 10–29 ml min−1), and severely impaired (group 4, requiring haemodialysis three times-a-week). Subjects received ziprasidone 40 mg day−1, given orally with food, as two divided daily doses for 7 days and a single 20 mg dose on the morning of day 8. Pharmacokinetic variables were determined from multiple venous blood samples collected on days 1 and 8 (haemodialysis day for subjects with severe renal impairment). Additional samples were collected from subjects with severe renal impairment on day 7 (nonhaemodialysis day).
Results
On day 1 there were no statistically significant differences in the pharmacokinetics (AUC(0, 12 h), Cmax, tmax) of ziprasidone among subjects with normal renal function and those with mild, moderate and severe renal impairment. The AUC(0,12 h) and Cmax in subjects with mildly impaired renal function were statistically significantly greater than in those with moderately impaired renal function (P = 0.0163–0.0385). The mean AUC(0,12 h) was 272, 370, 250 and 297 ng ml−1 h in groups 1, 2, 3 and 4, respectively. Corresponding mean Cmax values were 47, 61, 41 and 50 ng ml−1 and corresponding mean tmax values were 5, 6, 5 and 5 h. On day 8 there were no statistically significant differences in the pharmacokinetics (AUC(0, 12 h), Cmax, tmax, λz, Fb) of ziprasidone among subjects with normal renal function and those with moderate or severe renal impairment. The AUC(0,12 h) in subjects with mild renal impairment was statistically significantly greater than those in the other three groups (P =0.0025–0.0221), but this was not considered clinically significant. The mean AUC(0,12 h) were 446, 650, 389 and 427 ng ml −1h in groups 1, 2, 3 and 4, respectively. Corresponding mean Cmax values were 68, 93, 54 and 70 ng ml−1, corresponding mean tmax values were 4, 5, 4 and 5 h and corresponding mean λz were 0.14, 0.11, 0.14 and 0.17 h−1. The mean percentage Fb was 99.84–99.88% across all groups and the mean t½,z ranged from 4.2 to 6.4 h. Comparison of the mean AUC(0,12 h) and Cmax values in subjects with severe renal impairment on day 7 with those on day 8 suggested that haemodialysis does not have a clinically significant effect on the pharmacokinetics of ziprasidone.
Conclusions
The findings of this study indicate that mild-to-moderate impairment of renal function does not result in clinically significant alteration of ziprasidone pharmacokinetics and therefore does not necessitate dose adjustment.
Keywords: haemodialysis, pharmacokinetics, renal impairment, ziprasidone
Introduction
Most ziprasidone is excreted in the urine as either of two inactive primary metabolites formed by the action of CYP3A4; ziprasidone-sulphoxide and ziprasidone-sulphone [1]. In the case of drugs or active metabolites that are substantially renally excreted, accumulation and toxicity can occur in renally impaired individuals unless care is taken to adjust carefully dosing regimens intended for those with normal renal function. The pharmacokinetics of ziprasidone do not vary significantly with age [2], although renal function decreases by approximately 1% per year [3], suggesting that modest degrees of renal impairment do not have a clinically significant impact on the pharmacokinetics of ziprasidone. However, as ziprasidone is likely to be used to treat psychotic individuals who have concurrent renal impairment, we investigated specifically the impact of varying levels of renal impairment, independently of age, on the pharmacokinetics of ziprasidone and whether ziprasidone is cleared via haemodialysis.
Methods
Subjects
Thirty-nine subjects (aged 18–73 years) with varying degrees of renal function (defined in terms of creatinine clearance and requirement for haemodialysis) were enrolled in the study. The study protocol was approved by an independent institutional review board at the study site and all subjects provided written informed consent.
The subjects were assigned to four groups according to their level of renal function: normal renal function (group 1, creatinine clearance > 70 ml min−1); mildly impaired renal function (group 2, creatinine clearance 30–60 ml min−1); moderately impaired renal function (group 3, creatinine clearance 10–29 ml min−1); and severely impaired renal function (group 4, requiring haemodialysis three times-a-week). Subjects in groups 1, 2 and 3 were matched by age (± 10 years) and weight (± 11 kg).
Protocol
This was an open-label, multicentre, multiple-dose study designed to compare the steady-state pharmacokinetics of ziprasidone in subjects with normal renal function with those in matched subjects with varying degrees of renal function.
Subjects received ziprasidone 40 mg day−1, given orally with food as two divided daily doses for 7 days, and a single 20 mg dose on the morning of day 8. Ziprasidone was given, with 50 ml of water, in the morning immediately after eating a standard breakfast, and approximately 12 h later, immediately after eating a standard dinner. All concomitant therapy was administered at least 2 h before ziprasidone dosing.
Subjects with severe renal impairment underwent haemodialysis three times-a-week, including days 1 and 8. On days 1 and 8, haemodialysis was performed 4 h after receiving ziprasidone.
Pharmacokinetic sampling
Venous blood samples for the determination of serum ziprasidone concentrations were collected immediately before and up to 12 h after the morning dose of ziprasidone on day 1, and immediately before and up to 96 h after the morning dose of ziprasidone on day 8. Samples were also taken before the morning dose of ziprasidone on days 2, 3, 4 and 5. In addition, before the administration of ziprasidone on the morning of day 8, a venous blood sample was taken for the determination of ziprasidone plasma protein binding. Venous blood samples were collected on day 7, before and up to 12 h after the morning dose of ziprasidone from the subjects with severe renal impairment only.
Pharmacokinetic assessments
Serum concentrations of ziprasidone were determined using a validated high-pressure liquid chromatography (h.p.l.c.) assay with solid-phase extraction and ultraviolet (u.v.) detection. The assay had a dynamic range of 1.0–250.0 ng ml−1[4]. Ziprasidone concentrations below the lower limit of quantification were assigned a value of 0 ng ml−1 in pharmacokinetic calculations.
The percentage plasma protein binding of ziprasidone (Fb) was determined by equilibrium dialysis and h.p.l.c. with u.v. detection. The assay had a 25 ng ml−1 lower limit of detection.
The maximum serum concentrations of ziprasidone (Cmax), and the earliest time at which Cmax occurred (tmax) were estimated directly from the experimental data. The area under the serum ziprasidone concentration–time curve from time zero to 12 h postdosing (AUC(0, 12 h)) was estimated using linear trapezoidal approximation. The terminal phase rate constant (λz) was estimated using least-squares regression analysis of the serum concentration–time data obtained during the log-linear phase. The mean t½,z was calculated as ln 2/mean λz. The percentage Fb of ziprasidone was calculated using the following equation:
 |
Where Fb =fraction of bound ziprasidone, Cpe =concentration of ziprasidone in plasma, Cbe =concentration of ziprasidone in buffer, Vpe =volume of plasma at equilibrium, Vpi =volume of plasma before haemodialysis [5].
Statistical evaluation
The pharmacokinetic parameters were summarized using descriptive statistics. Geometric means and standard deviations were calculated for AUC(0,12 h) and Cmax. Arithmetic means and standard deviations were calculated for tmax, λz, and percentage Fb. Harmonic means were calculated for t½,z. The least-squares means statement in PROC GLM of SAS® was used to estimate the means and their variances, and an analysis of variance (anova) was performed to test for group effects. If a group effect was identified, Student’s t-tests were performed to test for differences between each group. In addition, for subjects with severe renal impairment, a pairwise Student’s t-test was used to compare AUC(0,12 h) on day 7 and day 8. A 5% level of significance was used throughout the study.
Results
Subjects
Thirty-nine subjects entered the study and 36 were evaluable for ziprasidone pharmacokinetics; nine in each group. The λz could not be estimated for one subject with moderate renal impairment (Group 3) due to insufficient data. All other subjects were included in the statistical comparisons of ziprasidone pharmacokinetics.
Pharmacokinetics
Mean serum ziprasidone concentrations for days 1 and 8 are shown as a function of time in Figures 1 and 2, respectively.
Figure 1.
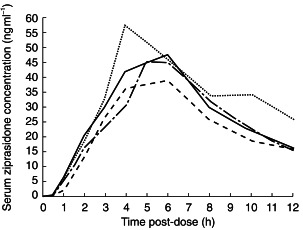
Mean serum ziprasidone concentrations on day 1 in subjects with normal (––), mildly impaired (-·-), moderately impaired (–––) and severely impaired (…..) renal function.
Figure 2.

Mean serum ziprasidone concentrations on day 8 in subjects with normal (––), mildly impaired (–·–), moderately impaired (–––) and severely impaired (…..) renal function.
On day 1 there were no statistically significant differences in the pharmacokinetics (AUC(0, 12 h), Cmax, tmax) of ziprasidone between subjects with normal renal function and the other three groups (Table 1). There were statistically significant differences between AUC(0,12 h) and Cmax for group 2 and those for group 3 (P = 0.0163–0.0385) (Table 2). Visual inspection of mean serum ziprasidone concentrations indicated that steady-state was reached by day 3 (data not shown).
Table 1.
Summary of pharmacokinetic parameters of ziprasidone on day 1.
| Pharmacokinetic parameter | Normal renal function (n = 9) | Mildly impaired renal function (n = 9) | Moderately impaired renal function (n = 9) | Severely impaired renal function (n = 9) |
|---|---|---|---|---|
| AUC(0,12 h)a (ng ml−1 h) | 272 ± 97 | 370 ± 145 | 250 ± 73 | 297 ± 75 |
| Cmaxa (ng ml−1) | 47 ± 20 | 61 ± 32 | 41 ± 11 | 50 ± 17 |
| tmaxb (h) | 5 ± 1 | 6 ± 2 | 5 ± 1 | 5 ± 2 |
Geometric means and s.d.
Arithmetic means and s.d.
Table 2.
Summary of 95% confidence intervals and P values for the pharmacokinetic parameters of ziprasidone on day 1.
| AUC(0,12 h) Ratio of geometric means (%) | Cmax Ratio of geometric means (%) | tmax | ||||
|---|---|---|---|---|---|---|
| Day 1 | 95% CI | P value | 95% CI | P value | 95% CI | P value |
| Normal vs mildly impaired renal function | 54,101 | 0.0558 | 52,112 | 0.1622 | −2,1 | 0.7709 |
| Normal vs moderately impaired renal function | 79,149 | 0.5848 | 78,169 | 0.4718 | −2,2 | 1.0 |
| Normal vs severely impaired renal function | 67,126 | 0.5806 | 64,138 | 0.7394 | −2,1 | 0.8842 |
| Mildly impaired vs moderately impaired renal function | 108,203 | 0.0163 | 102,222 | 0.0385 | −1,2 | 0.7709 |
| Mildly impaired vs severely impaired renal function | 91,171 | 0.1633 | 84,181 | 0.2816 | −1,2 | 0.8842 |
| Moderately impaired vs severely impaired renal function | 61,115 | 0.2752 | 56,120 | 0.2954 | −2,1 | 0.8842 |
CI = confidence interval.
On day 8 there was a 46–67% increase in mean AUC(0,12 h) in group 2 (mild renal impairment) compared with the other three groups (Table 3). There were statistically significant differences between the AUC(0,12 h) for group 2 and those for the other three groups (P = 0.0025–0.0221) (Table 4). Similarly, there was a 32–72% increase in the mean Cmax in group 2 compared with the other three groups (Table 3). There was a statistically significant difference between Cmax for group 2 and that for group 3 (moderate renal impairment (P = 0.0022)) but not for the other two groups (Table 4). When individual AUC(0,12 h) were plotted against baseline creatinine clearance, there were no apparent trends relating increasing ziprasidone exposure with decreasing renal function (Figure 3). There were no statistically significant differences between the four groups for tmax, or λz. The mean t½,z for groups 1–4 were 5.0, 6.4, 4.9 and 4.2 h, respectively. There was no apparent difference in percentage Fb between the four groups.
Table 3.
Summary of pharmacokinetic parameters of ziprasidone on day 8.
| Pharmacokinetic parameter | Normal renal function (n = 9) | Mildly impaired renal function (n = 9) | Moderately impaired renal function (n = 9) | Severely impaired renal function (n = 9) |
|---|---|---|---|---|
| AUC(0,12 h)a (ng ml−1 h) | 446 ± 145d | 650 ± 174def | 389 ± 111e | 427 ± 183f |
| Cmaxa (ng ml−1) | 68 ± 26 | 93 ± 28g | 54 ± 16e | 70 ± 29 |
| tmaxb (h) | 4 ± 1 | 5 ± 2 | 4 ± 1 | 5 ± 1 |
| λz (h−1) | 0.138 ± 0.044 | 0.108 ± 0.042 | 0.141 ± 0.050 | 0.166 ± 0.067 |
| t½,zc (h) | 5.0 | 6.4 | 4.9 | 4.2 |
| Fb (% bound) | 99.88 ± 0.043 | 99.84 ± 0.152 | 99.87 ± 0.058 | 99.86 ± 0.063 |
Geometric means and s.d.
Arithmetic means and s.d.
Calculated as ln 2/mean λz.
Statistically significant difference between mildly impaired renal function and normal renal function, P =0.0221.
Statistically significant difference between mildly impaired renal function and moderately impaired renal function, P =0.0025.
Statistically significant difference between mildly impaired renal function and severely impaired renal function, P =0.0113.
Statistically significant difference between mildly impaired renal function and moderately impaired renal function, P =0.0022.
Table 4.
Summary of 95% confidence intervals (CI) and P values for the pharmacokinetic parameters of ziprasidone on day 8.
| AUC(0,12 h) Ratio of geometric means (%) | Cmax Ratio of geometric means (%) | tmax | ||||
|---|---|---|---|---|---|---|
| Day 8 | 95% CI | P value | 95% CI | P value | 95% CI | P value |
| Normal vs mildly impaired renal function | 50,94 | 0.0221 | 53,103 | 0.0724 | −2,1 | 0.4705 |
| Normal vs moderately impaired renal function | 83,158 | 0.3857 | 91,179 | 0.1520 | −2,1 | 0.7721 |
| Normal vs severely impaired renal function | 76,144 | 0.7784 | 70,138 | 0.9291 | −2,1 | 0.3143 |
| Mildly impaired vs moderately impaired renal function | 122,230 | 0.0025 | 124,243 | 0.0022 | −1,2 | 0.6642 |
| Mildly impaired vs severely impaired renal function | 111,210 | 0.0113 | 96,188 | 0.0865 | −2,1 | 0.7721 |
| Moderately impaired vs severely impaired renal function | 66,125 | 0.5556 | 55,108 | 0.1292 | −2,1 | 0.4705 |
Figure 3.

Comparison of individual creatinine clearance with ziprasidone AUC(0,12 h) values on day 8 in subjects with normal (□), mildly (•), moderately (◊) and severely impaired (▴) renal function. Asterisks indicate the mean values for each renal function group.
Comparison of the pharmacokinetics of ziprasidone in subjects in group 4 (severe renal impairment) on day 7 (nonhaemodialysis day) with those on day 8 (haemodialysis day) revealed considerable intersubject variability. There was a small increase in AUC(0,12 h) and Cmax (both < 40%) leading to a statistically significant difference for the AUC(0,12 h) (P =0.0258) (Table 5). Individual AUC(0,12 h) values on days 7 and 8 are shown in Figure 4.
Table 5.
Effect of haemodialysis on the pharmacokinetics of ziprasidone in subjects with severely impaired renal function.
| Pharmacokinetic parameter | Non-haemodialysis day (Day 7) (n = 9) | Haemodialysis day (Day 8) (n = 9) |
|---|---|---|
| AUC(0,12 h)a (ng ml−1 h) | 334 ± 84a | 427 ± 183a |
| Cmaxa, c (ng ml−1) | 51 ± 10 | 70 ± 29 |
| tmaxb, d (h) | 4 ± 2 | 5 ± 1 |
Geometric means and s.d.
Arithmetic means and s.d.
Statistically significant difference between day 7 and day 8, P =0.0258.
Statistical comparison between days not performed.
Figure 4.

Effect of haemodialysis on the AUC(0,12 h) of ziprasidone in subjects with severely impaired renal function.
Discussion
This open-label, multicentre, multiple-dose study, involving 36 subjects with varying degrees of renal function, evaluated the influence of renal impairment on the pharmacokinetics of ziprasidone. The principal findings from this study indicate that mild-to-moderate renal impairment, defined by subnormal creatinine clearance, does not result in clinically significant alterations in the pharmacokinetics of ziprasidone at steady state.
There were no statistically significant differences between subjects with normal renal function and the other three groups in the three pharmacokinetic parameters assessed on day 1 (AUC(0, 12 h), Cmax, tmax), suggesting that mild-to-moderate renal impairment does not influence the first-dose pharmacokinetics of ziprasidone. The AUC(0,12 h) and Cmax values for the group with mildly impaired renal function tended to be higher than those in the other three groups, including those with more severe renal impairment. However, this difference only reached significance between subjects with mildly impaired and moderately impaired renal function, but was not considered clinically significant.
On day 8 there were no statistically significant differences among the groups with moderate and severe renal impairment and the group with normal renal function in the five pharmacokinetic parameters that were compared in statistical tests (AUC(0, 12 h), Cmax, tmax, λz, (Fb). There were, however, statistically significant differences between the group with mild renal impairment and the other three groups with respect to the AUC(0,12 h) and between the group with mild renal impairment and the group with moderate renal impairment with respect to the Cmax. The mean AUC(0,12 h) in the group with mild renal impairment was 45–67% higher than the corresponding values for the other three groups, and mean Cmax in the group with mild renal impairment was 37% higher than that in the group with normal renal function.
The reason for the relatively high mean AUC(0,12 h) value in the group with mild renal impairment compared with the other groups is unknown, but may be an artefact resulting from the relatively small number of subjects studied. In this study, in the groups with normal, mildly impaired, moderately impaired and severely impaired renal function, AUC(0,12 h) values ranged from 247 to 830, 386–851, 239–525 and 209–785 ng ml−1h, respectively. As such, there was considerable overlap in the AUC(0,12 h) values between the groups. Furthermore, theoretical considerations do not provide a basis upon which increased exposure to ziprasidone might be expected to occur in subjects with mild renal impairment but not in those with more severe renal impairment. For these reasons, the difference in the AUC(0,12 h) for the group with mild renal impairment compared with the other groups is not considered to be clinically significant. Similarly, the relatively high mean Cmax value in the group with mild renal impairment is unlikely to be clinically important because there was also considerable overlap in the Cmax values between the groups. Thus, in the groups with normal, mildly impaired, moderately impaired and severely impaired renal function these values ranged from 30 to 111, 59–131, 34–80, and 34–123 ng ml−1, respectively.
The high degree of overlap in the AUC(0,12 h) and the Cmax between the groups in this study is in accord with expectations. As changes in total drug clearance are only proportional to renal function (defined in terms of creatinine clearance) for drugs solely excreted unchanged, and less than 1% of an oral dose of ziprasidone is excreted unchanged in the urine, randomization of subjects on the basis of their level of renal function is unlikely to result in groups with discrete ziprasidone pharmacokinetic profiles [1, 3]. The findings of this study support this supposition and suggest that one or more factor(s) distinct from renal function determines the pharmacokinetics of ziprasidone.
The observation of similar Fb values across all four groups is noteworthy because concentrations of α1-acid glycoprotein and human serum albumin can change in renal disease [6]. Even in the subjects with severe renal impairment, the percentage Fb for ziprasidone was almost identical to that in subjects with normal renal function (99.86%vs 99.88%). This absence of altered binding of ziprasidone to plasma proteins provides evidence to suggest that systemic exposure to ziprasidone in subjects with renal impairment may be correlated with central D2 receptor occupancy, as it is in healthy subjects [7]. The absence of altered binding of ziprasidone to plasma proteins in subjects with renal impairment also has implications in the context of haemodialysis. As might be expected for a drug that is highly bound to plasma proteins, haemodialysis did not appear to have a clinically significant effect on the pharmacokinetics of ziprasidone in this study. Although the AUC(0,12 h) for ziprasidone on day 8 (haemodialysis day) was statistically significantly different from that on day 7 (nonhaemodialysis day) (P =0.0258), it was actually greater on day 8 than on day 7. Thus, although the monohydrate of ziprasidone HCl has a molecular weight of 467 and is highly water-soluble [8], ziprasidone is not removed by haemodialysis, probably because it is highly protein-bound. The reason for the apparent increase in AUC(0,12 h) on day 8 is unknown but may be attributable to suboptimal blood and dialysate flow rates. It should be noted, however, that there was considerable overlap between the AUC(0,12 h) values in the subjects with severely impaired renal function on day 7 (204–458 ng ml−1h) and those on day 8 (209–785 ng ml−1 h).
As most of the newer antipsychotics undergo extensive presystemic elimination and are predominantly cleared via hepatic elimination, renal function is not usually taken into consideration when therapeutic dosing strategies using these drugs are being devised [9]. The findings of this study suggest that this strategy may also be appropriate for ziprasidone. Despite the existence of similarities between ziprasidone and some of the newer antipsychotics, the pharmacokinetic profile of ziprasidone in individuals with renal impairment appears to have the potential to confer some practical clinical benefits. Most notable in this respect is the comparison with risperidone. Although renal clearance accounts for less than 10% of the total clearance of risperidone, it accounts for approximately 40% of the total clearance of its principal active metabolite, 9-hydroxy-risperidone, and renal impairment has been shown to have a substantial effect on the pharmacokinetics of orally administered risperidone and 9-hydroxy-risperidone [10]. In one study, the AUC(0, ∞), Cmax and total clearance of risperidone were statistically significantly different in subjects with moderate renal impairment (mean creatinine clearance 44 ml min−1) compared with healthy, young subjects (aged 30 ± 4 years). More importantly, it also demonstrated statistically significant differences, with respect to several pharmacokinetic parameters for both risperidone and 9-hydroxy-risperidone, between subjects with moderate renal impairment or severe renal impairment (mean creatinine clearance 17 ml min−1) and healthy young subjects [10]. For these reasons, careful dose titration of risperidone is advised in patients with renal disease.
In conclusion, the findings of this study indicate that mild-to-moderate renal impairment does not influence the steady-state pharmacokinetics of ziprasidone. This suggests that ziprasidone dose adjustment on the basis of renal function is not required.
References
- 1.Prakash C, Kamel A, Cui D, et al. Identification of the major human liver cytochrome P450 isoform responsible for the primary metabolites of ziprasidone and prediction of possible drug interactions. Br J Clin Pharmacol. 2000;49(Suppl. 1):35S–42S. doi: 10.1046/j.1365-2125.2000.00151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wilner KD, Tensfeldt TG, Baris B, et al. Single- and multiple-dose pharmacokinetics of ziprasidone in healthy young and elderly volunteers. Br J Clin Pharmacol. 2000;49(Suppl. 1):15S–20S. doi: 10.1046/j.1365-2125.2000.00148.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Morrison RBI, Davidson JM, Kerr DNS. Clinical physiology of the kidney. Tests of renal function and structure. In: Weatherall DJ, Ledingham JGG, Warrell DA, editors. Oxford Textbook of Medicine. 2. Oxford University Press; 1989. pp. 18.2–18.17. [Google Scholar]
- 4.Janiszewski JS, Fouda HG, Cole RO. Development and validation of a high-sensitivity assay for an antipsychotic agent, CP-88,059, with solid-phase excavation and narrow-bore high-performance liquid chromatography. J Chromatogr. 1995;668:133–139. doi: 10.1016/0378-4347(95)00071-p. [DOI] [PubMed] [Google Scholar]
- 5.Boudinot FD, Jusko WJ. Fluid shifts and other factors affecting plasma protein binding of prednisolone by equilibrium dialysis. J Pharm Sci. 1984;6:774–780. doi: 10.1002/jps.2600730617. [DOI] [PubMed] [Google Scholar]
- 6.Pacifici GM, Viani A, Taddeucci-brunelli G, et al. Effects of development, ageing and renal and hepatic insufficiency as well as hemodialysis on the plasma concentrations of albumin and α1-acid glycoprotein: implications for binding of drugs. Ther Drug Monit. 1986;8:259–293. doi: 10.1097/00007691-198609000-00003. [DOI] [PubMed] [Google Scholar]
- 7.Bench CJ, Lammertsma AA, Grasby PM, et al. The time course of binding to striatal dopamine D2 receptors by the neuroleptic ziprasidone (CP-88, 059, 01) determined by positron emission tomography. Psychopharmacology. 1996;124:141–147. doi: 10.1007/BF02245614. [DOI] [PubMed] [Google Scholar]
- 8.Howard HR, Prakash C, Seeger TF. Ziprasidone hydrochloride. Drugs Future. 1994;19:560–563. [Google Scholar]
- 9.De Vane CL. Brief comparison of the pharmacokinetics and pharmacodynamics of the traditional and newer antipsychotic drugs. Am J Health Syst Pharm. 1995;52(Suppl 1):S15–S18. doi: 10.1093/ajhp/52.3_Suppl_1.S15. [DOI] [PubMed] [Google Scholar]
- 10.Snoeck E, Van Peer A, Sack M, et al. Influence of age, renal and liver impairment on the pharmacokinetics of risperidone in man. Psychopharmacology. 1995;122:223–229. doi: 10.1007/BF02246543. [DOI] [PubMed] [Google Scholar]


