Abstract
Aims
To evaluate the pharmacokinetics and tolerability of single and multiple oral doses of ziprasidone in healthy male volunteers, and to determine the influence of ziprasidone on serum prolactin levels.
Methods
Single and multiple doses of ziprasidone were given orally (as two divided daily doses), at fixed dosages of 10 and 40 mg day−1, and using titrated regimens of 40–80 and 40–120 mg day−1, for 14 days. All dosages were taken immediately after food. The study adopted a randomized, double-blind, placebo-controlled design. Prolactin response, sedative properties, tolerability, and extrapyramidal symptoms were also investigated.
Results
Steady-state exposure to ziprasidone was attained after 1 day of dosing. Mean Cmax and AUC(0,12 h) increased with increasing dose, with apparent dose-proportionality between the 20 and 60 mg dose levels. Trough-to-peak ratios at steady state ranged from 2 to 5. Accumulation ratios for the fixed-dose regimens were 1.49 and 1.48 at the 5 and 20 mg dose levels, respectively. Ziprasidone was associated with transient prolactin elevation but levels of prolactin returned to baseline within the dosing interval at steady state. There was a marginal, transient increase in serum prolactin levels which was not dose-related at the 80 and 120 mg day−1 doses, and which was noted to attenuate with chronic dosing. Ziprasidone was generally well tolerated. The most frequent side-effect was mild or moderate headache. A minority of patients suffered first-dose postural hypotension. Ziprasidone was also associated with a mild sedative effect that became less pronounced as treatment continued. There were no drug-related changes in electrocardiogram or clinical laboratory variables that were of clinical importance.
Conclusions
Ziprasidone is characterized by a predictable pharmacokinetic profile resulting in symptoms that reflect its pharmacological action.
Keywords: extrapyramidal symptoms, pharmacokinetics, serum prolactin, ziprasidone
Introduction
The unique combination of pharmacological activities of ziprasidone predicts a broad range of beneficial effects, as well as a low liability for inducing movement disorders. Laboratory and clinical findings have led to the hypothesis that preferential antagonism of central 5-HT2A receptors by ziprasidone limits its potential to induce movement disorders, and improves its efficacy against positive and negative symptoms of schizophrenia [1]. This profile appears to offer a potential advantage over other medications such as risperidone because clinical experience with this drug suggests that clinical efficacy in the absence of extrapyramidal symptoms is limited to a narrow dose range for most patients (4–6 mg day−1) [2].
The present study was undertaken to evaluate the pharmacokinetics and tolerability of single and multiple oral dosages of ziprasidone in healthy male volunteers. The effect of ziprasidone on serum prolactin levels was also investigated.
Methods
Subjects
The study involved 39 healthy male volunteers aged 18–45 years, whose body weights were between 61 and 91 kg, and who were within 10% of the ideal weight for age and height [3]. All were nonsmokers. Each had WBC, neutrophil count, MCH, BUN, and creatinine levels within 10% of the normal range, and haemoglobin and haematocrit within 5% of the normal range. The subjects were also required to have urinalysis, glucose-6-phosphate dehydrogenase, MCV, SGOT, SGPT, and alkaline phosphatase values within normal limits, and bilirubin < 10% above the upper limit of normal. Blood pressure was required to be between 95/65 and 140/90 mmHg, and heart rate between 50 and 100 beats min−1.
Subjects with any condition thought likely to affect the absorption of ziprasidone were excluded, as were those with clinical evidence or history of allergic, haematological, renal, endocrine, pulmonary, gastrointestinal, cardiovascular, hepatic, psychiatric or neurological disease, including all forms of epilepsy. Subjects with known drug or alcohol dependence or drug allergies were also excluded.
None of the subjects had taken prescription, over-the-counter or recreational drugs for at least 2 weeks before entering the study and none had received an investigational drug for at least 4 weeks.
The study protocol was approved by an independent institutional review board and all subjects provided informed written consent.
Study design
In this double-blind, parallel-group study, subjects were randomised to receive placebo or one of four doses of ziprasidone: 10 mg day−1, 40 mg day−1, 40 mg day−1 escalated to 80 mg day−1, and 40 mg day−1 escalated to 120 mg day−1. All dosages were given as two divided daily doses.
Earlier trials had indicated that dosage escalation of ziprasidone might minimize the risk of syncope and/or postural hypotension. Dosage escalation was therefore used in this study, but was discontinued if any of the following reactions to the initial dose occurred: adverse side-effects or reactions; clinical laboratory abnormalities; decrease in RBC, WBC, neutrophils or haemoglobin attributable to ziprasidone; significant electrocardiogram (ECG) abnormalities such as symptomatic dysrhythmias requiring therapy; 2nd or 3rd degree A–V block, or a temperature greater than 38°C.
In all groups, the first dose was administered on the morning of day 1, and was followed by a 48 h postdose washout to obtain blood samples for determination of single-dose pharmacokinetic data. The 80 and 120 mg day−1 groups received a single 20 mg dose on day 1. From days 4–17 medication was given twice daily (every 12 h) and the final dose was given on the morning of day 18. For the subjects undergoing dose escalation, the dose of ziprasidone was increased to 80 mg day−1 on day 7 and, in one group, to 120 mg day−1 on day 10. After the final dose of ziprasidone on the morning of day 18, all subjects received single-blind placebo until the final assessments on day 20.
Doses were administered with 50 ml water immediately after a standard breakfast or a standard evening meal (eaten over 20 min).
Subjects were asked to refrain from lying down (except for vital sign measurements), and from eating or drinking caffeinated beverages during the first 4 h postdose on the mornings of days 1 and 18. Subjects stayed at the clinical research facility under continuous medical supervision for at least 36 h before the first dose, and until 72 h after the last dose on day 18.
Pharmacokinetic assessments
Blood samples (8 ml, sufficient to provide 3 ml of serum) for pharmacokinetic analysis were collected on days 1 and 18 immediately before dosing and 0.5, 1, 2, 3, 4, 6, 8, 12, 18, 24, 36, 48, and 72 h after dosing. Additional samples were collected immediately before dosing on days 5–17 to determine trough serum drug levels. All samples were collected in evacuated tubes that were free of preservative and anticoagulant and were stored at room temperature until clotting had occurred. Within 1 h after collection, the serum was separated from whole blood in a refrigerated centrifuge and stored at −20°C until analysis.
Serum concentrations of ziprasidone were determined by high performance liquid chromatography (h.p.l.c.) using liquid–liquid extraction and an atmospheric pressure ionization mass spectrometry (API-MS) detection method [4]. The lower limit of quantification for the assay was 0.5 ng ml−1 and the upper limit was 50 ng ml−1. The assay was linear and accurate within 5% over this range. Serum concentrations below the lower limit were assigned a value of 0 ng ml−1 for the estimation of pharmacokinetic parameters.
The maximum observed serum concentration (Cmax) and the time at which it occurred (tmax) were estimated directly from individual plasma concentration–time curves. The terminal phase rate constant, λz, was estimated using least-squares regression analysis of the curve during the terminal log-linear phase. The mean terminal phase half-life, t½,z, was calculated as ln 2/mean λz.
The area under the concentration–time curve from time zero to time t of the last sample with quantifiable concentrations of ziprasidone (AUC(0, t)) was estimated using linear trapezoidal approximation. The area under the concentration–time curve from time t to infinity (AUC(t,∞)) was estimated as Cpest /λz, where Cpest was the estimated concentration at time t based upon the regression analysis. Area under the serum concentration–time curve from time 0 to infinity (AUC(0, ∞)) was estimated as the sum of AUC(0, t) and AUC(t,∞). Area under the curve from time 0–12 h postdose, AUC(0,12 h), was also estimated using linear trapezoidal approximation.
For the two groups in which there was no dose escalation, the accumulation ratio (R) was determined from the ratio of the day 18 AUC(0,12 h)/day 1 AUC(0,12 h). Similarly, the predicted accumulation ratio after the first dose (R′) was estimated from the ratio of the day 1 AUC(0,∞)/day 1 AUC(0,12 h).
Serum prolactin
Blood samples for the determination of serum prolactin concentrations were collected 0.25 and 0.5 h before the first dose of ziprasidone or placebo on day 1. Additional samples were collected at the same times as the pharmacokinetic samples. Serum was separated from whole blood as described above. Serum prolactin was assayed using standard methods.
Tolerability
Sedation was evaluated by subject self-report. In total, 12 categories related to sedation (drowsy, sleepy, slowed down, sedated, tired, worn out, listless, fatigued, exhausted, sluggish, weary, bushed) were scored using a scale of 0 (absent) to 7 (severe). Sedation profiles were undertaken: within 24 h before dosing on day 1 at 0.5, 1, 2, 3, 4, 6, 8, 12, 24 and 48 h after the first dose; prior to morning and evening dosage on days 4–17; prior to and 0.5, 1, 2, 3, 4, 6, 8, 12, 24, 48, 72, 96 and 120 h postdose on day 18. The subjects also completed a standardized sleep profile self-rating scale immediately after waking on days 1–23. Eleven questions related to sleep were evaluated by means of an analogue scale (e.g. with extremes of ‘not at all’ to ‘definitely’). The subjects also indicated how long they had taken to fall asleep, the duration of uninterrupted sleep and the incidence of waking during the night.
Extrapyramidal symptoms were evaluated using the Simpson–Angus rating scale [5]. Each subject was scored on a scale of 0 (absent) to 4 (extreme) for each of the following 10 categories: gait, arm dropping, shoulder shaking, elbow rigidity, fixation of position or wrist rigidity, leg pendulousness, head dropping, glabella tap, tremor, and salivation.
Subjects were observed and questioned regarding the presence of akathisia according to the method of Barnes [6]. For this assessment, the signs and symptoms of various categories of akathisia observed by the investigator were scored on a scale of 0 (none) to 3 (severe) with the subject in the sitting, standing, and lying positions.
A global evaluation based on the Abnormal Involuntary Movement Scale (AIMS) was also undertaken in which abnormal movements, incapacitation due to abnormal movements, and the subject’s awareness of abnormal movements were scored by the investigator on a scale of 0 (none) to 4 (severe) [7].
These three assessments were undertaken at the following times: within 24 h of the first dose of trial medication; 2, 12, and 24 h postdose on day 1; prior to administration of the morning dose on days 4, 6, 8, 10, 12, 14, and 16; and 0, 2, 12, 24, 48, 72, 96, and 120 h postdose on day 18.
All observed or volunteered adverse events occurring up to 6 days after the last dose were recorded together with details of onset, duration, severity (mild, moderate, severe), treatment required, and an assessment of the possible relationship to study treatment. The events were classified by body system using the preferred terms of the COSTART system.
Routine clinical laboratory tests (haematology and clinical chemistry) were undertaken at the screening visit, immediately prior to administration of ziprasidone or placebo on days 1, 4, 10 and 18; 24 h postdose on day 1 and 72 h postdose on day 18. Additional liver function tests were performed on day 0 (within 24 h prior to the first dose of ziprasidone or placebo); and at 24 and 48 h after dosing on day 18. Dipstick urinalysis was undertaken with microscopic evaluation of the sediment.
Standing and supine blood pressures and pulse rates were measured at approximately the same time as the clinical laboratory assessments. A standard 12-lead ECG was obtained for each subject at screening; at 0, 1, 4, 10, 18 and 24 h postdose on day 1; and 72 h after the morning dose on day 18. Oral temperature was recorded at the same time.
Statistical analysis
There were no formal statistical analyses. Cmax, AUC(0,12 h) and AUC(0,∞) were measured by geometric means and standard deviations. Arithmetic means and standard deviations were calculated for all other parameters except t½,z. Changes in serum prolactin at each time point on days 1 and 18 were described using means and standard deviations. Extrapyramidal side-effects including akathisia and Parkinsonian symptoms, AIMS scores, sedation and sleep ratings were summarized descriptively.
Results
Subjects
A total of 39 subjects entered the study. Baseline characteristics were similar in each of the five treatment groups (Table 1).
Table 1.
Baseline demographic data
| Ziprasidone daily dosage | |||||
|---|---|---|---|---|---|
| 10 mg (n =6) | 40 mg (n = 8) | 40–80 mg (n = 8) | 40–120 mg (n = 7) | Placebo (n = 10) | |
| Age (years) | |||||
| Mean | 23.8 | 29.5 | 25.1 | 30.8 | 26.2 |
| Range | (20–29) | (20–45) | (18–34) | (22–40) | (20–34) |
| Weight (kg) | |||||
| Mean | 74.9 | 76.3 | 76.3 | 74.9 | 79.1 |
| Range | (63–93) | (61–93) | (68–86) | (67–88) | (62–96) |
| Race | |||||
| Black | 2 | 4 | 3 | 3 | 3 |
| White | 4 | 4 | 4 | 4 | 7 |
| Other | 0 | 0 | 1 | 0 | 0 |
Two subjects in the 40 mg day−1 group and one subject in the 40–80 mg day−1 group discontinued the study as a result of treatment-related postural hypotension. In the 40 mg day−1 group, postural hypotension followed the initial dose of ziprasidone on day 1. In the 40–80 mg day−1 group, postural hypotension was observed on day 9. An additional six subjects, four from the placebo group and one each from the 40–80 mg day−1 and 40–120 mg day−1 groups, discontinued for reasons not related to treatment. All those who discontinued ziprasidone were excluded from the pharmacokinetic evaluation at day 18 but were included in the evaluation on day 1.
Single-dose pharmacokinetics
Mean Cmax following a single 5 mg dose of ziprasidone was 12.2 ng ml−1 compared with values between 26.6 and 60.0 ng ml−1 in the three groups receiving ziprasidone at an initial dose of 20 mg (Table 2). Mean tmax values in the 10 and 40 mg day−1 dose groups were similar, with values ranging from approximately 4–5 h. The serum concentration–time profiles for the four treatment groups are shown in Figure 1.
Table 2.
Summary of pharmacokinetic parameters (mean±s.d.) following single (5 and 20 mg day−1) and multiple (10, 40, 80 and 120 mg day−1) doses of ziprasidone in healthy male subjects in the fed state.
| Ziprasidone | ||||
|---|---|---|---|---|
| 10 mg day−1 | 40 mg day−1 | 40–80 mg day−1 | 40–120 mg day−1 | |
| Day 1 | ||||
| Number of subjects | 6 | 8 | 8 | 7 |
| Dose (mg) | 5 | 20 | 20 | 20 |
| Cmax (ng ml−1) | 12.2 ± 4.1 | 26.6 ± 18.9 | 60.0 ± 33.8 | 34.3 ± 13.1 |
| tmax (h) | 5.0 ± 1.1 | 4.8 ± 1.3 | 3.8 ± 1.8 | 4.0 ± 1.7 |
| AUC(0,12 h) (ng ml−1 h) | 73.7 ± 18.7 | 175.7 ± 134.0 | 314.6 ± 166.8 | 215.0 ± 77.1 |
| AUC(0, ∞) (ng ml−1 h) | 86.7 ± 22.1 | 226.3 ± 174.8 | 376.7 ± 190.9 | 308.4 ± 125.9 |
| λz (h−1) | 0.220 ± 0.048 | 0.143 ± 0.049 | 0.172 ± 0.034 | 0.161 ± 0.040 |
| t½,z (h)a | 3.2 | 4.8 | 4.0 | 4.3 |
| Predicted accumulation ratio | 1.18 ± 0.08 | 1.29 ± 0.10 | – | – |
| Day 18 | ||||
| Number of subjects | 6 | 6 | 6 | 6 |
| Final dose (mg day−1) | 10 | 40 | 80 | 120 |
| Cmax (ng ml−1) | 14.8 ± 6.7 | 44.6 ± 48.1 | 118.6 ± 80.1 | 139.4 ± 81.2 |
| tmax (h) | 5.2 ± 1.3 | 3.8 ± 2.2 | 3.7 ± 0.8 | 4.7 ± 1.5 |
| AUC(0,12 h) (ng ml−1 h) | 109.8 ± 46.7 | 259.2 ± 213.3 | 658.0 ± 334.6 | 1027.9 ± 446.8 |
| λz (h−1) | 0.175 ± 0.054 | 0.145 ± 0.027 | 0.079 ± 0.040 | 0.069 ± 0.049 |
| t½,z (h)a | 4.0 | 4.8 | 8.8 | 10.0 |
| Accumulation ratio | 1.49 ± 0.39 | 1.48 ± 0.67 | – | – |
Calculated as ln 2/mean λz.
Figure 1.
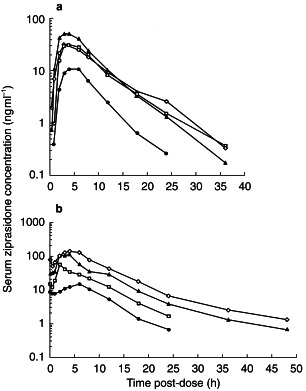
Serum concentration–time profiles following administration of single (a) and multiple (b) oral doses of ziprasidone to healthy male volunteers immediately after food. Dosages of ziprasidone were 10 mg day−1 (•), 40 mg day−1 (□), 40–80 mg day−1 (▴) and 40–120 mg day−1 (◊).
Mean λz values were similar across all four groups, ranging from 0.143 to 0.220 h−1 and corresponded to mean half-lives between 3.2 and 4.8 h. Mean AUC(0,12 h) was 73.7 ng ml−1 h after the 5 mg dose and between 175.7 and 314.6 ng ml−1h in the groups that received a 20 mg dose. The predicted geometric mean accumulation ratios were 1.18 for the single 5 mg dose and 1.29 at the single 20 mg dose.
Multiple-dose pharmacokinetics
Trough serum concentrations between days 5 and 17 suggested that steady-state conditions were attained within 1 day of dosing. Mean Cmax was higher in all dose groups on day 18 than on day 1 (Table 2). Day 18 Cmax values were dose-related, ranging from 14.8 ng ml−1 in the 10 mg day−1 group to 139.4 ng ml−1 in the 120 mg day−1 group (Table 2; Figure 1). The rate of absorption was similar in all groups and appeared unchanged following multiple dosing as indicated by similar values for tmax on days 1 and 18 (Table 2). In addition, steady-state normalized Cmax values were similar in all four groups.
Drug exposure estimated by AUC(0,12 h) increased with dose from a mean of 109.8 ng ml −1h in the 10 mg day−1 group to 1027.9 ng ml −1h in the 120 mg day−1 group. With the exception of the 10 mg day−1 regimen, steady-state AUC(0,12 h) normalized for dose increased with increasing dose. At the end of the dosing period the observed geometric mean accumulation ratios for the 10 mg day−1 and 40 mg day−1 groups were 1.49 and 1.48, respectively (Table 2).
As shown in Table 2, the mean terminal phase elimination rate constant was smaller in the 80 mg day−1 and 120 mg day−1 groups on day 18 than in the 10 mg day−1 or 40 mg day−1 groups. This was related to the appearance of an additional disposition phase. The harmonic mean terminal phase half-lives ranged from 4 to 5 h in the 10 mg day−1 and 40 mg day−1 groups to 8–10 h in the 80 mg day−1 and and 120 mg day−1 groups. The peak-to-trough concentration ratios generally ranged from 2 to 5 across all dose groups.
Serum prolactin concentrations
With both single and chronic dosing, ziprasidone was associated with a transient, marginal elevation in serum prolactin which returned to baseline within the dosing interval at all ziprasidone doses (Table 3). Maximum prolactin elevations associated with ziprasidone occurred 2–6 h postdose, corresponding with tmax for ziprasidone plasma concentrations.
Table 3.
Serum prolactin concentrations (ng ml−1) (change from baseline).
| Ziprasidone daily dosage | |||||
|---|---|---|---|---|---|
| 10 mg day−1 | 40 mg day−1 | 40–80 mg day−1 | 40–120 mg day−1 | Placebo | |
| Baseline | |||||
| Prolactin | 10.47 | 12.14 | 9.88 | 9.63 | 9.91 |
| Number of subjects | 6 | 8 | 8 | 7 | 10 |
| Day 1 | |||||
| Number of subjects | 6 | 8 | 8 | 7 | 10 |
| Ziprasidone dose (mg) | 5 | 20 | 20 | 20 | |
| Maximum postdose prolactin | 21.57 | 36.14 | 37.56 | 30.56 | 15.9 |
| (+ 11.10) | (+ 24.00) | (+ 27.68) | (+ 20.93) | (+ 5.28) | |
| 12 h postdose prolactin | 10.99 | 12.58 | 8.29 | 10.87 | 10.56 |
| (+ 0.52) | (+ 0.44) | (−1.59) | (+ 1.24) | (+ 0.65) | |
| Day 18 | |||||
| Number of subjects | 6 | 6 | 6 | 6 | 6 |
| Ziprasidone dose (mg day−1) | 10 | 40 | 80 | 120 | |
| Maximum postdose prolactin | 18.24 | 22.64 | 38.75 | 30.01 | 15.81 |
| (+ 7.77) | (+ 10.50) | (+ 28.87) | (+ 20.38) | (+ 5.90) | |
| 12 h postdose prolactin | 7.04 | 5.66 | 6.68 | 11.55 | 10.03 |
| (−3.43) | (−6.48) | (−3.20) | (+ 1.92) | (+ 0.12) | |
Maximal elevations with ziprasidone reported at 2–6 h postdose. Normal limits for men for the assay used=3–14.7 ng ml−1.
There was no evidence of a dose-relationship at 80 or 120 mg day−1 as shown by the magnitude of the maximum postdose elevation, which was actually lower for 120 mg day−1 than 80 mg day−1 on day 18. Plasma concentration–time curves for these two doses on day 18 confirm that while serum ziprasidone concentrations were dose-related, serum prolactin concentrations were not.
In addition, there appeared to be some attenuation of the transient prolactin elevation with multiple ziprasidone dosing compared with single dosing. With multiple ziprasidone dosing there was pharmacokinetic accumulation, as shown by the increase in AUC(0,12 h) normalized for dose, between days 1 and 18 in the 10 mg day−1 and 40 mg day−1 groups. However, this accumulation was not matched by proportionate increases in serum prolactin levels between days 1 and 18 at these doses.
Sedation and sleep profiles
Ziprasidone was associated with mild sedation compared with placebo following the administration of single 5 and 20 mg doses on day 1. The peak mean scores (i.e. the mean summated score from 7-point assessments in 12 different categories, where a maximum score of 84 would imply extreme sedation) were 17.7, 15.4, 22.6 and 21.3 in the 10, 40, 40–80 and 40–120 mg day−1 groups, respectively. Sedation occurred earlier with the single 20 mg dose (3 h) than with the single 5 mg dose (8 h). Mean sedation scores on day 18 were similar to day 1 baseline scores and placebo in the 10, 40, 40–80 and 40–120 mg day−1 groups (Figure 2). The 40–120 mg day−1 group experienced mild sedation (peak mean score 25.8) between 2 and 8 h postdose.
Figure 2.
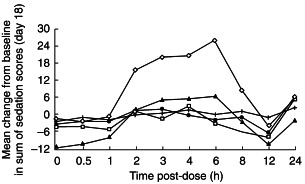
Mean change from baseline in sum of sedation scores on day 18 profiles following administration of multiple oral doses of ziprasidone 10 mg day−1 (•), 40 mg day−1 (□), 40–80 mg day−1 (▴) and 40–120 mg day−1 (◊), or placebo (+), to healthy male volunteers immediately after food.
The sleep profile remained relatively stable for all dose groups throughout the treatment period. However, at the end of double-blind treatment with ziprasidone at dosages ≥ 40 mg day−1, the mean time to fall asleep increased by between 70 and 84 min and the mean duration of sleep decreased from baseline values of 6.11–6.36 h by between 2.25 h and 3.93 h.
Adverse events
Thirty-one of the 39 subjects who entered the study reported a total of 112 adverse events (Table 4). Similar numbers of subjects in each of the five treatment groups experienced adverse events although the total number of adverse events was higher in the ziprasidone 40, 40–80 and 40–120 mg day−1 groups compared with placebo. The most frequently occurring adverse event was headache. This was generally mild or moderate in severity, except for two subjects in the 40 mg day−1 group. One subject had severe headache on days 11 and 16, which began 10.9 and 9.8 h after ziprasidone administration and lasted 4.3 and 12.5 h, respectively. The other subject had severe headache on day 3 that began 55.3 h after initial dosing and lasted 7.2 h.
Table 4.
Summary of treatment-emergent adverse events
| Ziprasidone daily dosage (mg day−1) | |||||
|---|---|---|---|---|---|
| 10 (n = 6) | 40 (n = 8) | 40–80 (n = 8) | 40–120 (n = 7) | Placebo (n = 10) | |
| Subjects with adverse events | 4 | 7 | 8 | 6 | 6 |
| Total adverse events | 9 | 22 | 43 | 28 | 10 |
| Most frequent adverse events | |||||
| Headache | 2 | 4 | 5 | 3 | 1 |
| Postural hypotension | 0 | 2 | 2 | 3 | 0 |
| Dizziness | 0 | 1 | 3 | 2 | 0 |
| Somnolence | 0 | 1 | 1 | 2 | 0 |
| Insomnia | 0 | 1 | 2 | 1 | 0 |
Nine subjects had Simpson–Angus scores of 1 (three in the 40 mg day−1 group, five in the 40–80 mg day−1 group and one in the 40–120 mg day−1 group) indicating the presence of mild extrapyramidal symptoms. Only one of the nine subjects reported tremor as an adverse event (on day 12, duration 48 h). One other subject reported tremor (day 14, duration 19 h), but his Simpson–Angus score remained at 0 for the duration of the study. No subject had an AIMS score or Barnes akathisia score above 0 at any time.
Seven subjects developed postural hypotension, and in three subjects the episodes were severe (two at 40–120 mg day−1 and one at 40 mg day−1). Two subjects with postural hypotension, both in the 40 mg day−1 group, were withdrawn from the study after the first dose of ziprasidone, one with moderate and one with severe symptoms. A third subject (40–80 mg day−1 group) was withdrawn after 9 days of treatment because of moderate postural hypotension whilst receiving ziprasidone at 80 mg day−1. Six subjects experienced dizziness which was severe in two subjects, and four subjects experienced somnolence. All severe episodes of dizziness, hypotension, or somnolence occurred on the first day of dosing, in four of the 23 subjects who received ziprasidone at 40 mg day−1.
A reduction in mean supine diastolic blood pressure was observed in the 40 mg day−1 group, and between days 10 and 18 this was more marked than in the other groups. The changes in diastolic blood pressure at higher dosages were not different from those after placebo. However, mean reductions from baseline in standing systolic blood pressure between 4 and 8 h postdose on day 1 were more marked in all ziprasidone groups than with placebo. This effect diminished during multiple dosing.
There were no clinically significant ECG findings related to treatment with ziprasidone, nor were there clinically important trends or abnormalities in any of the laboratory test variables.
Discussion
The objective of the present study was to evaluate the pharmacokinetics and tolerability of a range of single and multiple oral doses of ziprasidone in order to help define a suitable dosage regimen for Phase III clinical trials. The effect of ziprasidone administration on serum prolactin levels was also evaluated. The rationale for administering ziprasidone after food was based on our previous unpublished studies (Pfizer Inc., data on file) that showed that the presence of food in the stomach increases oral bioavailability. Steady-state conditions were generally attained after 1 day of dosing over the 10–120 mg day−1 dose range.
The overall exposure to ziprasidone at steady-state appeared dose-proportional between 40 and 120 mg day−1 based on dose-adjusted mean AUC(0,12 h) values. The adjusted AUC(0,12 h) values for the 10 mg day−1 dose level appeared to be disproportionately large. The relationship between Cmax and dose was similar to that observed for AUC(0,12 h).
Multiple-dose administration resulted in increased pharmacokinetic variability compared with single doses. This was inferred from increases in coefficients of variation for Cmax and AUC(0,12 h) between days 1 and 18. However, the degree of pharmacokinetic variability was not dose-dependent. Individual steady-state values ranged approximately three- to seven-fold but intersubject variability was as high as 20-fold in the 40 mg day−1 group.
At steady-state, the terminal half-life at the 80 and 120 mg day−1 dosage levels was longer (9–10 h) than that observed with the 10–40 mg day−1 dosages (4–5 h). The longer half-lives were related to the detection of an additional disposition phase which only became apparent following multiple dosing; this finding was not related to a dose-dependent decrease in oral clearance. The observed accumulation at the 10 and 40 mg day−1 dose levels was greater than that predicted by first-dose parameters of 30% and 24%, respectively. This may, in part, be related to an underestimation of AUC(0,∞) at low doses due to an inability to characterize the additional disposition phase.
At clinically effective doses (80 and 120 mg day−1), ziprasidone was not associated with sustained prolactin elevation in men. Although there was a marginal, transient elevation in prolactin, this was not dose-related at the 80 and 120 mg day−1 doses with levels returning to baseline within the dosing interval. In addition, the slight increase in maximum serum prolactin concentration was not apparent 12 h after dosing at steady state.
The lack of sustained prolactin elevation with ziprasidone is in contrast to the effect of older neuroleptic agents such as haloperidol, which is associated with marked and sustained prolactin elevation as a consequence of very potent D2 receptor antagonism [8]. This also contrasts with the newer antipsychotic agent, risperidone, which is also associated with hyperprolactinaemia [9–15]. The slight, transient prolactin elevation associated with ziprasidone in this study is similar to that observed with clozapine [16] which, in general, does not induce clinically significant hyperprolactinaemia [17, 18].
The lack of sustained hyperprolactinaemia with ziprasidone may reflect its relatively short plasma elimination half-life of approximately 6–8 h because the pituitary lactotrophs, which are responsible for prolactin release, are situated outside the blood–brain barrier and may not therefore be exposed to ziprasidone for prolonged periods [19, 20]. If ziprasidone is cleared less rapidly from the central nervous system than from the plasma, this may explain why ziprasidone has sufficient pharmacodynamic activity at central D2 receptors to reduce psychotic symptoms but does not cause hyperprolactinaemia. In addition, the constellation of serotonergic activities of ziprasidone (i.e. very potent antagonism at 5-HT2A receptors, potent antagonism at 5-HT2C and 5-HT1D receptors, and agonist activity at 5-HT1A receptors) may also contribute to the lack of hyperprolactinaemia.
The adverse effects of sustained hyperprolactinaemia include sexual dysfunction, particularly in men [21, 22], which is a major cause of medication noncompliance in patients with schizophrenia [23]. In addition, hyperprolactinaemia is also associated with galactorrhoea, gynaecomastia and amenorrhoea [24], and possibly with decreased bone mineral density and subsequent increased risk of fracture [25, 26]. The fact that ziprasidone does not induce hyperprolactinaemia in men is encouraging as this may translate into improved tolerability and compliance compared with conventional neuroleptics and some of the newer antipsychotic agents. Long-term clinical trials will further evaluate this aspect of ziprasidone’s activity.
The most frequently occurring adverse event was mild or moderate headache. A minority of subjects experienced adverse events rated as severe, including headache, orthostatic hypotension, somnolence and dizziness. These were predominantly first-dose effects and reflect experience from preliminary pharmacokinetic studies (Pfizer Inc., data on file). Orthostatic hypotension is thought to reflect α1-adrenoceptor blockade. In this respect ziprasidone has been shown to have lesser propensity to produce orthostatic hypotension than that of other antipsychotic agents, including olanzapine, quetiapine and sertindole [27].
None of the subjects displayed a maximum Simpson-Angus score of > 1 and most maintained a score of zero throughout the trial despite steady-state administration of therapeutic doses. In all cases, extrapyramidal symptoms were mild or absent, and there was no evidence of dose-dependency. Moreover, no positive AIMS scores or akathisia determinations were noted for any subject. These findings confirm the predicted low incidence of movement disorders with ziprasidone on the basis of its potent antagonism at 5-HT2 receptors [1].
Although single doses of ziprasidone were associated with an increase in sedation scores which reached a peak at tmax, a relevant sedative effect was noted only in the 40–120 mg day−1 group on the last day of dosing, providing additional evidence that tolerance develops on repeated dosing. In support of this hypothesis, it has been proposed that sedative effects caused by new antipsychotic agents are related to antagonism of central H1 receptors, and in comparative studies ziprasidone has been shown to have less affinity for H1 receptors than do risperidone, olanzapine and remoxipride [26]. In 4- and 6-week clinical trials, ziprasidone has been shown to be associated with somnolence, which was generally of mild or moderate severity, and which attenuated during the studies [28].
In summary, the results of this study indicate that ziprasidone has a predictable pharmacokinetic profile characterized by rapid attainment of steady-state and dose-proportional Cmax and AUC(0,12 h). Systemic ziprasidone exposure following 14 days of multiple dosing increases with doses ranging between 10 and 120 mg day−1, and is associated with symptoms consistent with the pharmacological properties of the drug.
References
- 1.Seeger TF, Seymour PA, Schmidt AW, et al. Ziprasidone (CP-88,059): a new antipsychotic with combined dopamine and serotonin receptor antagonist activity. J Pharmacol Exp Ther. 1995;275:101–113. [PubMed] [Google Scholar]
- 2.Bersani G, Bressa GM, Meco G, et al. Combined serotonin 5HT2 and dopamine D2 antagonism in schizophrenia: clinical. Extrapyramidal and neuroendocrine response in a preliminary study with risperidone (R 64766) Hum Psychopharmacol. 1990;5:225–231. [Google Scholar]
- 3.Metropolitan height and weight tables Stat Bull Metrop Life Found. 1983;64:3–9. [PubMed] [Google Scholar]
- 4.Janiszewski JS, Fouda HG, Cole RO. Development and validation of a high-sensitivity assay for an antipsychotic agent, CP-88,059, with solid-phase extraction and narrow-bone, high-performance liquid chromatography. J Chromatogr. 1995;668:133–139. doi: 10.1016/0378-4347(95)00071-p. [DOI] [PubMed] [Google Scholar]
- 5.Simpson GM, Angus JW. A rating scale for extrapyramidal side effects. Acta Psychiatr Scand. 1970;212(Suppl):11–19. doi: 10.1111/j.1600-0447.1970.tb02066.x. [DOI] [PubMed] [Google Scholar]
- 6.Barnes TRE. A rating scale for drug-induced akathisia. Br J Psychiatr. 1989;154:672–676. doi: 10.1192/bjp.154.5.672. [DOI] [PubMed] [Google Scholar]
- 7.Guy W. Abnormal Involuntary Movement Scale. In: Guy W, editor. ECDEU Assessment Manual for Psychopharmacology. Maryland, USA: Department of Health and Human Services Publication (ADM-76–338); 1976. [Google Scholar]
- 8.Rubin RT. Prolactin and schizophrenia. In: Meltzer HY, editor. Psychopharmacology: the Third Generation of Progress. New York: Raven Press; 1984. pp. 803–808. [Google Scholar]
- 9.Mesotten F, Suy E, Pietquin M, et al. Therapeutic effect and safety of increasing doses of risperidone (R 64766) in psychotic patients. Psychopharmacology. 1989;99:445–459. doi: 10.1007/BF00589890. [DOI] [PubMed] [Google Scholar]
- 10.Muller-spahn F. Risperidone in the treatment of chronic schizophrenic patients: an international double-blind, parallel-group study versus haloperidol. Clin Neuropharmacol. 1992;15(1A):825–835. doi: 10.1097/00002826-199201001-00048. [DOI] [PubMed] [Google Scholar]
- 11.DeCoster R, Bowden C, Byloos M, et al. Endocrine effects of the new antipsychotic risperidone. Presented at the 9thInternational Congress of Endocrinology, Nice, France.
- 12.Claus A, Bollen J, De Cuyper H, et al. Risperidone versus haloperidol in the treatment of chronic schizophrenic inpatients: a multicentre, double-blind, comparative study. Acta Psychiatr Scand. 1992;85:295–305. doi: 10.1111/j.1600-0447.1992.tb01473.x. [DOI] [PubMed] [Google Scholar]
- 13.Ereshefsky L, Lacombe S. Pharmacological profile of risperidone. Can J Psychiatry. 1993;38:S80–S88. [PubMed] [Google Scholar]
- 14.Cardoni AA. Risperidone: review and assessment of its role in the treatment of schizophrenia. Ann Pharmacother. 1995;29:610–618. doi: 10.1177/106002809502900611. [DOI] [PubMed] [Google Scholar]
- 15.Umbricht D, Kane JM. Risperidone: efficacy and safety. Schizophr Bull. 1995;21:593–606. doi: 10.1093/schbul/21.4.593. [DOI] [PubMed] [Google Scholar]
- 16.Lee HS, Kim CH, Song SH, Choi NK, Yoo KJ. Clozapine does not elevate serum prolactin levels in healthy men. Biol Psychiatry. 1995;38:762–764. doi: 10.1016/0006-3223(95)00366-5. [DOI] [PubMed] [Google Scholar]
- 17.Meltzer HY. The mechanism of action of novel antipsychotic drugs. Schizophr Bull. 1991;17:263–287. doi: 10.1093/schbul/17.2.263. [DOI] [PubMed] [Google Scholar]
- 18.Meltzer HY. Treatment of neuroleptic non-responsive schizophrenic patients. Schizophr Bull. 1992;18:515–542. doi: 10.1093/schbul/18.3.515. [DOI] [PubMed] [Google Scholar]
- 19.Muller EE, Locatelli V, Cella S, Penalva A, Novelli A, Cocchi D. Prolactin-lowering and releasing drugs. Drugs. 1983;25:399–432. doi: 10.2165/00003495-198325040-00004. [DOI] [PubMed] [Google Scholar]
- 20.De Koning P, de Vries MH. A comparison of the neuro-endocrinological and temperature effects of DU 29894, flesinoxan, sulpiride and haloperidol in normal volunteers. Br J Clin Pharmacol. 1995;39:7–14. doi: 10.1111/j.1365-2125.1995.tb04403.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rojansky N, Wang K, Halbreich U. Adverse Effects of Psychotropic Drugs. Guildford Press: 1992. Reproductive and sexual adverse effects on psychotropic drugs; pp. 356–375. [Google Scholar]
- 22.Schwartz MF, Bauman JE, Masters WH. Hyperprolactinaemia and sexual disorders in men. Biol Psychiatry. 1982;17:861–876. [PubMed] [Google Scholar]
- 23.Fleischhacker WW, Meise U, Gunther V, et al. Compliance with antipsychotic drug treatment: influence of side effects. Acta Psychiatr Scand. 1994;89:11–15. [PubMed] [Google Scholar]
- 24.Buvat J, Lemaire A, Buvat-herbaut M, et al. Hyperprolactinemia and sexual function in men. Hormone Res. 1985;22:196–203. doi: 10.1159/000180094. [DOI] [PubMed] [Google Scholar]
- 25.Ataya K, Mercado A, Kartiaginer J, et al. Bone density and reproductive hormones in patients with neuroleptic-induced hyperprolactinemia. Fertil Steril. 1988;50:876–881. doi: 10.1016/s0015-0282(16)60365-5. [DOI] [PubMed] [Google Scholar]
- 26.Halbreich U, Rojansky N, Palter S, et al. Decreased bone mineral density in medicated psychiatric patients. Psychosomatic Med. 1995;57:485–491. doi: 10.1097/00006842-199509000-00011. [DOI] [PubMed] [Google Scholar]
- 27.Pickar D. Prospects for pharmacotherapy of schizophrenia. Lancet. 1995;345:557–562. doi: 10.1016/s0140-6736(95)90469-7. [DOI] [PubMed] [Google Scholar]
- 28.Tandon R, Harrigan E, Zorn SH. Ziprasidone: a novel antipsychotic with unique therapeutic potential. J Serotonin Res. 1997;4:159–177. [Google Scholar]


