Abstract
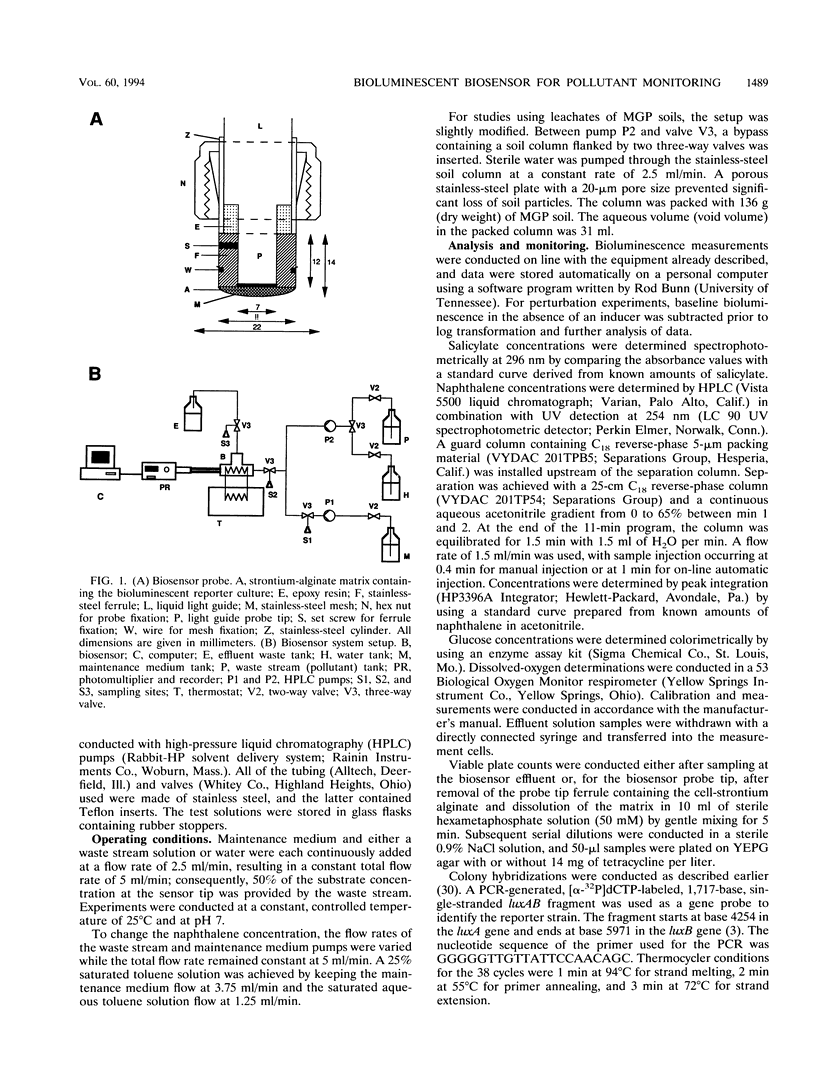
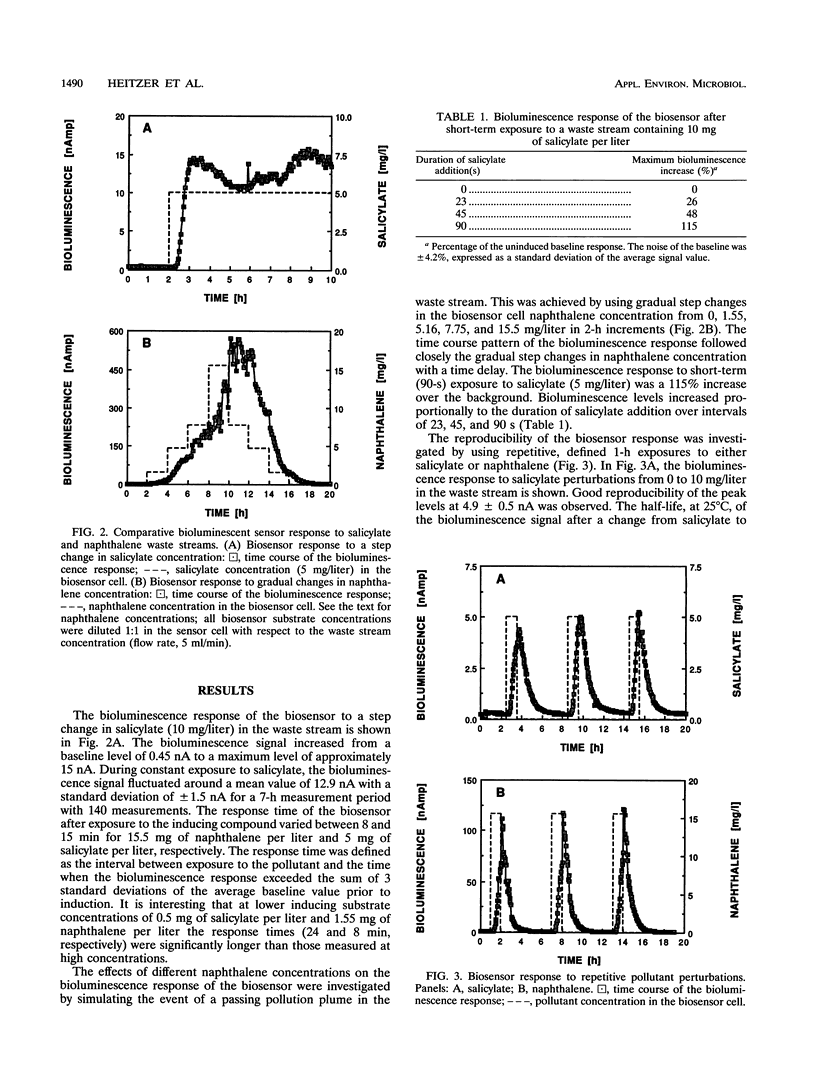
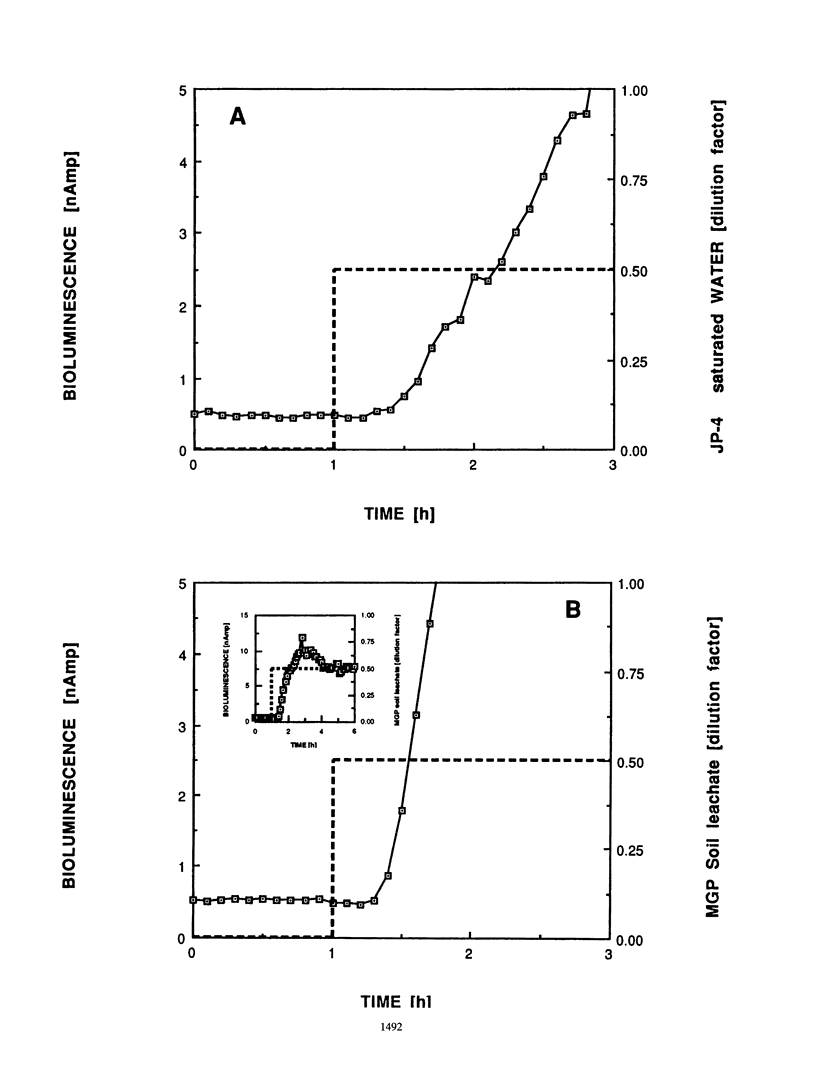
An optical whole-cell biosensor based on a genetically engineered bioluminescent catabolic reporter bacterium was developed for continuous on-line monitoring of naphthalene and salicylate bioavailability and microbial catabolic activity potential in waste streams. The bioluminescent reporter bacterium, Pseudomonas fluorescens HK44, carries a transcriptional nahG-luxCDABE fusion for naphthalene and salicylate catabolism. Exposure to either compound resulted in inducible bioluminescence. The reporter culture was immobilized onto the surface of an optical light guide by using strontium alginate. This biosensor probe was then inserted into a measurement cell which simultaneously received the waste stream solution and a maintenance medium. Exposure under defined conditions to both naphthalene and salicylate resulted in a rapid increase in bioluminescence. The magnitude of the response and the response time were concentration dependent. Good reproducibility of the response was observed during repetitive perturbations with either naphthalene or salicylate. Exposure to other compounds, such as glucose and complex nutrient medium or toluene, resulted in either minor bioluminescence increases after significantly longer response times compared with naphthalene or no response, respectively. The environmental utility of the biosensor was tested by using real pollutant mixtures. A specific bioluminescence response was obtained after exposure to either an aqueous solution saturated with JP-4 jet fuel or an aqueous leachate from a manufactured-gas plant soil, since naphthalene was present in both pollutant mixtures.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnold M. A. Fiber-optic biosensors. J Biotechnol. 1990 Aug;15(3):219–228. doi: 10.1016/0168-1656(90)90028-a. [DOI] [PubMed] [Google Scholar]
- Atlas R. M., Sayler G., Burlage R. S., Bej A. K. Molecular approaches for environmental monitoring of microorganisms. Biotechniques. 1992 May;12(5):706–717. [PubMed] [Google Scholar]
- Baldwin T. O., Devine J. H., Heckel R. C., Lin J. W., Shadel G. S. The complete nucleotide sequence of the lux regulon of Vibrio fischeri and the luxABN region of Photobacterium leiognathi and the mechanism of control of bacterial bioluminescence. J Biolumin Chemilumin. 1989 Jul;4(1):326–341. doi: 10.1002/bio.1170040145. [DOI] [PubMed] [Google Scholar]
- Burlage R. S., Sayler G. S., Larimer F. Monitoring of naphthalene catabolism by bioluminescence with nah-lux transcriptional fusions. J Bacteriol. 1990 Sep;172(9):4749–4757. doi: 10.1128/jb.172.9.4749-4757.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham C. R., Leslie D., Squirrell D. J. Gene probe assays on a fibre-optic evanescent wave biosensor. Biosens Bioelectron. 1992;7(7):487–493. doi: 10.1016/0956-5663(92)80005-v. [DOI] [PubMed] [Google Scholar]
- Hastings J. W., Potrikus C. J., Gupta S. C., Kurfürst M., Makemson J. C. Biochemistry and physiology of bioluminescent bacteria. Adv Microb Physiol. 1985;26:235–291. doi: 10.1016/s0065-2911(08)60398-7. [DOI] [PubMed] [Google Scholar]
- Heitzer A., Mason C. A., Hamer G. Heat shock gene expression in continuous cultures of Escherichia coli. J Biotechnol. 1992 Jan;22(1-2):153–169. doi: 10.1016/0168-1656(92)90139-z. [DOI] [PubMed] [Google Scholar]
- Heitzer A., Webb O. F., Thonnard J. E., Sayler G. S. Specific and quantitative assessment of naphthalene and salicylate bioavailability by using a bioluminescent catabolic reporter bacterium. Appl Environ Microbiol. 1992 Jun;58(6):1839–1846. doi: 10.1128/aem.58.6.1839-1846.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karube I., Matsunaga T., Mitsuda S., Suzuki S. Microbial electrode BOD sensors. Biotechnol Bioeng. 1977 Oct;19(10):1535–1547. doi: 10.1002/bit.260191010. [DOI] [PubMed] [Google Scholar]
- King J. M., Digrazia P. M., Applegate B., Burlage R., Sanseverino J., Dunbar P., Larimer F., Sayler G. S. Rapid, sensitive bioluminescent reporter technology for naphthalene exposure and biodegradation. Science. 1990 Aug 17;249(4970):778–781. doi: 10.1126/science.249.4970.778. [DOI] [PubMed] [Google Scholar]
- Meighen E. A. Molecular biology of bacterial bioluminescence. Microbiol Rev. 1991 Mar;55(1):123–142. doi: 10.1128/mr.55.1.123-142.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menn F. M., Applegate B. M., Sayler G. S. NAH plasmid-mediated catabolism of anthracene and phenanthrene to naphthoic acids. Appl Environ Microbiol. 1993 Jun;59(6):1938–1942. doi: 10.1128/aem.59.6.1938-1942.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rattray E. A., Prosser J. I., Killham K., Glover L. A. Luminescence-based nonextractive technique for in situ detection of Escherichia coli in soil. Appl Environ Microbiol. 1990 Nov;56(11):3368–3374. doi: 10.1128/aem.56.11.3368-3374.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruby E. G., Hastings J. W. Formation of hybrid luciferases from subunits of different species of Photobacterium. Biochemistry. 1980 Oct 28;19(22):4989–4993. doi: 10.1021/bi00563a009. [DOI] [PubMed] [Google Scholar]
- Sanseverino J., Applegate B. M., King J. M., Sayler G. S. Plasmid-mediated mineralization of naphthalene, phenanthrene, and anthracene. Appl Environ Microbiol. 1993 Jun;59(6):1931–1937. doi: 10.1128/aem.59.6.1931-1937.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayler G. S., Layton A. C. Environmental application of nucleic acid hybridization. Annu Rev Microbiol. 1990;44:625–648. doi: 10.1146/annurev.mi.44.100190.003205. [DOI] [PubMed] [Google Scholar]
- Sayler G. S., Shields M. S., Tedford E. T., Breen A., Hooper S. W., Sirotkin K. M., Davis J. W. Application of DNA-DNA colony hybridization to the detection of catabolic genotypes in environmental samples. Appl Environ Microbiol. 1985 May;49(5):1295–1303. doi: 10.1128/aem.49.5.1295-1303.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheper T. Biosensors for process monitoring. J Ind Microbiol. 1992 May-Jun;9(3-4):163–172. doi: 10.1007/BF01569620. [DOI] [PubMed] [Google Scholar]
- Selifonova O., Burlage R., Barkay T. Bioluminescent sensors for detection of bioavailable Hg(II) in the environment. Appl Environ Microbiol. 1993 Sep;59(9):3083–3090. doi: 10.1128/aem.59.9.3083-3090.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw J. J., Dane F., Geiger D., Kloepper J. W. Use of bioluminescence for detection of genetically engineered microorganisms released into the environment. Appl Environ Microbiol. 1992 Jan;58(1):267–273. doi: 10.1128/aem.58.1.267-273.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart G. S., Williams P. lux genes and the applications of bacterial bioluminescence. J Gen Microbiol. 1992 Jul;138(7):1289–1300. doi: 10.1099/00221287-138-7-1289. [DOI] [PubMed] [Google Scholar]
- Yen K. M., Serdar C. M. Genetics of naphthalene catabolism in pseudomonads. Crit Rev Microbiol. 1988;15(3):247–268. doi: 10.3109/10408418809104459. [DOI] [PubMed] [Google Scholar]
- de Weger L. A., Dunbar P., Mahafee W. F., Lugtenberg B. J., Sayler G. S. Use of Bioluminescence Markers To Detect Pseudomonas spp. in the Rhizosphere. Appl Environ Microbiol. 1991 Dec;57(12):3641–3644. doi: 10.1128/aem.57.12.3641-3644.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]



