Abstract
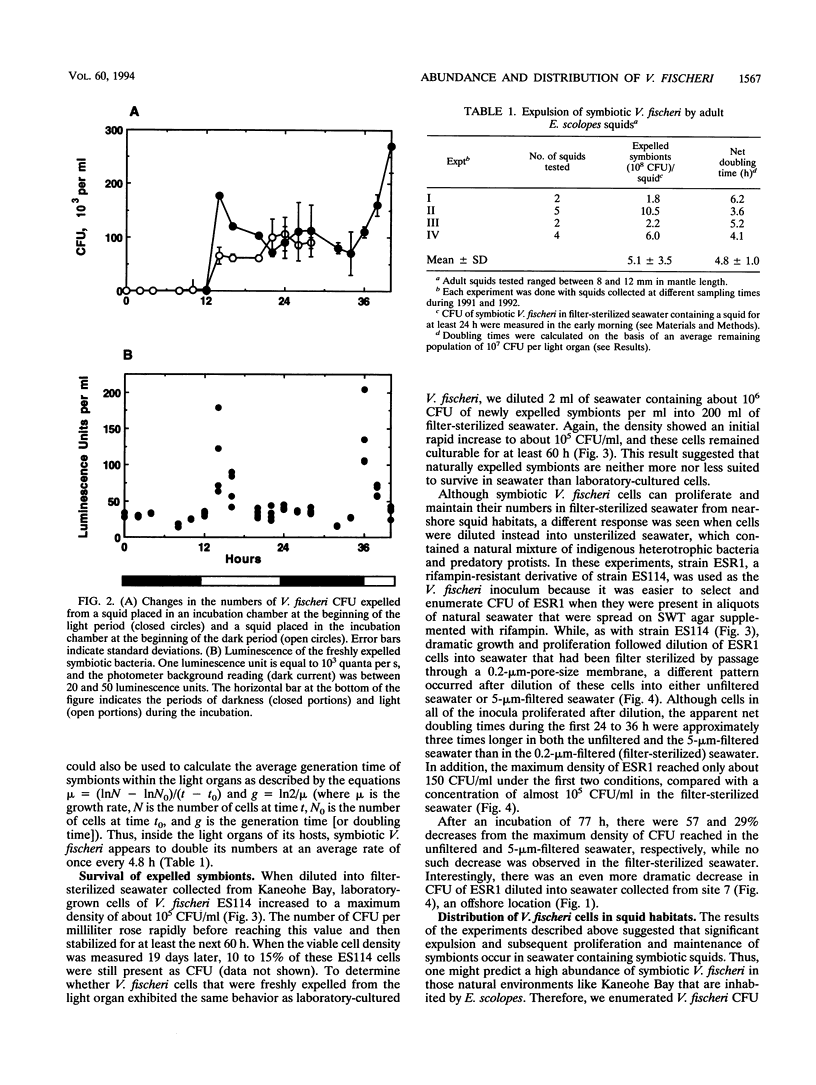
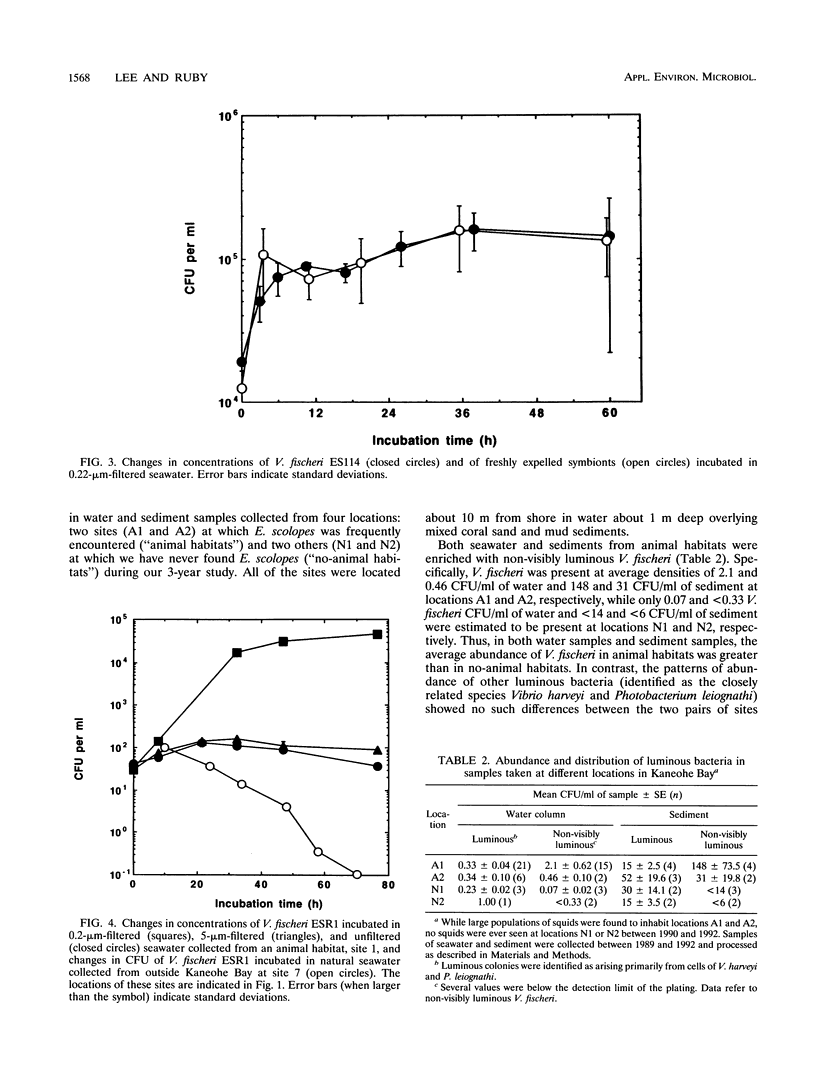
Euprymna scolopes, a Hawaiian species of bioluminescent squid, harbors Vibrio fischeri as its specific light organ symbiont. The population of symbionts grew inside the adult light organ with an average doubling time of about 5 h, which produced an excess of cells that were expelled into the surrounding seawater on a diurnal basis at the beginning of each period of daylight. These symbionts, when expelled into the ambient seawater, maintain or slightly increase their numbers for at least 24 h. Hence, locations inhabited by their hosts periodically receive a daily input of symbiotic V. fischeri cells and, as a result, become significantly enriched with these bacteria. As estimated by hybridization with a species-specific luxA gene probe, the typical number of V. fischeri CFU, both in the water column and in the sediments of E. scolopes habitats, was as much as 24 to 30 times that in similar locations where squids were not observed. In addition, the number of symbiotic V. fischeri CFU in seawater samples that were collected along a transect through Kaneohe Bay, Hawaii, decreased as a function of the distance from a location inhabited by E. scolopes. These findings constitute evidence for the first recognized instance of the abundance and distribution of a marine bacterium being driven primarily by its symbiotic association with an animal host.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boettcher K. J., Ruby E. G. Depressed light emission by symbiotic Vibrio fischeri of the sepiolid squid Euprymna scolopes. J Bacteriol. 1990 Jul;172(7):3701–3706. doi: 10.1128/jb.172.7.3701-3706.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray K. M., Greenberg E. P. Physical and functional maps of the luminescence gene cluster in an autoinducer-deficient Vibrio fischeri strain isolated from a squid light organ. J Bacteriol. 1992 Jul;174(13):4384–4390. doi: 10.1128/jb.174.13.4384-4390.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K. H., Ruby E. G. Detection of the Light Organ Symbiont, Vibrio fischeri, in Hawaiian Seawater by Using lux Gene Probes. Appl Environ Microbiol. 1992 Mar;58(3):942–947. doi: 10.1128/aem.58.3.942-947.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makemson J. C., Fulayfil N., Basson P. Association of luminous bacteria with artificial and natural surfaces in arabian gulf seawater. Appl Environ Microbiol. 1992 Jul;58(7):2341–2343. doi: 10.1128/aem.58.7.2341-2343.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFall-Ngai M. J., Ruby E. G. Symbiont recognition and subsequent morphogenesis as early events in an animal-bacterial mutualism. Science. 1991 Dec 6;254(5037):1491–1494. doi: 10.1126/science.1962208. [DOI] [PubMed] [Google Scholar]
- Orndorff S. A., Colwell R. R. Distribution and identification of luminous bacteria from the sargasso sea. Appl Environ Microbiol. 1980 May;39(5):983–987. doi: 10.1128/aem.39.5.983-987.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roszak D. B., Colwell R. R. Survival strategies of bacteria in the natural environment. Microbiol Rev. 1987 Sep;51(3):365–379. doi: 10.1128/mr.51.3.365-379.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruby E. G., Asato L. M. Growth and flagellation of Vibrio fischeri during initiation of the sepiolid squid light organ symbiosis. Arch Microbiol. 1993;159(2):160–167. doi: 10.1007/BF00250277. [DOI] [PubMed] [Google Scholar]
- Ruby E. G., Greenberg E. P., Hastings J. W. Planktonic marine luminous bacteria: species distribution in the water column. Appl Environ Microbiol. 1980 Feb;39(2):302–306. doi: 10.1128/aem.39.2.302-306.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruby E. G., McFall-Ngai M. J. A squid that glows in the night: development of an animal-bacterial mutualism. J Bacteriol. 1992 Aug;174(15):4865–4870. doi: 10.1128/jb.174.15.4865-4870.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simek K., Chrzanowski T. H. Direct and indirect evidence of size-selective grazing on pelagic bacteria by freshwater nanoflagellates. Appl Environ Microbiol. 1992 Nov;58(11):3715–3720. doi: 10.1128/aem.58.11.3715-3720.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wimpee C. F., Nadeau T. L., Nealson K. H. Development of species-specific hybridization probes for marine luminous bacteria by using in vitro DNA amplification. Appl Environ Microbiol. 1991 May;57(5):1319–1324. doi: 10.1128/aem.57.5.1319-1324.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woomer P., Singleton P. W., Bohlool B. B. Ecological indicators of native rhizobia in tropical soils. Appl Environ Microbiol. 1988 May;54(5):1112–1116. doi: 10.1128/aem.54.5.1112-1116.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]



