Abstract
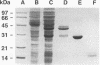
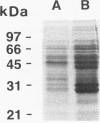
Bacteria of two species, Escherichia coli and Corynebacterium glutamicum, were used as hosts to express recombinant ovine gamma interferon as a fusion protein with glutathione S-transferase. The recombinant gamma interferon produced by both bacteria was biologically active in vitro and was recognized by anti-gamma interferon monoclonal antibodies. E. coli produced large amounts of soluble recombinant protein which could be purified by a simple affinity chromatography method. Only a small fraction of the recombinant protein made by C. glutamicum was recovered by this method. Expression of recombinant protein in C. glutamicum was unstable but could be controlled by increased regulation of the tac promoter. Both hosts expressed ovine gamma interferon at high levels, with the recombinant protein making up a significant proportion of the cellular protein content.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Booth R. J., Harris D. P., Love J. M., Watson J. D. Antigenic proteins of Mycobacterium leprae. Complete sequence of the gene for the 18-kDa protein. J Immunol. 1988 Jan 15;140(2):597–601. [PubMed] [Google Scholar]
- Calos M. P. DNA sequence for a low-level promoter of the lac repressor gene and an 'up' promoter mutation. Nature. 1978 Aug 24;274(5673):762–765. doi: 10.1038/274762a0. [DOI] [PubMed] [Google Scholar]
- Derynck R., Singh A., Goeddel D. V. Expression of the human interferon-gamma cDNA in yeast. Nucleic Acids Res. 1983 Mar 25;11(6):1819–1837. doi: 10.1093/nar/11.6.1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Entrican G., McInnes C. J., Rothel J. S., Haig D. M. Kinetics of ovine interferon-gamma production: detection of mRNA and characterisation of biological activity. Vet Immunol Immunopathol. 1992 Jun;33(1-2):171–178. doi: 10.1016/0165-2427(92)90044-q. [DOI] [PubMed] [Google Scholar]
- Gray P. W., Leung D. W., Pennica D., Yelverton E., Najarian R., Simonsen C. C., Derynck R., Sherwood P. J., Wallace D. M., Berger S. L. Expression of human immune interferon cDNA in E. coli and monkey cells. Nature. 1982 Feb 11;295(5849):503–508. doi: 10.1038/295503a0. [DOI] [PubMed] [Google Scholar]
- Kemp D. J., Coppel R. L., Cowman A. F., Saint R. B., Brown G. V., Anders R. F. Expression of Plasmodium falciparum blood-stage antigens in Escherichia coli: detection with antibodies from immune humans. Proc Natl Acad Sci U S A. 1983 Jun;80(12):3787–3791. doi: 10.1073/pnas.80.12.3787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Liebl W., Sinskey A. J., Schleifer K. H. Expression, secretion, and processing of staphylococcal nuclease by Corynebacterium glutamicum. J Bacteriol. 1992 Mar;174(6):1854–1861. doi: 10.1128/jb.174.6.1854-1861.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundell D., Lunn C., Dalgarno D., Fossetta J., Greenberg R., Reim R., Grace M., Narula S. The carboxyl-terminal region of human interferon gamma is important for biological activity: mutagenic and NMR analysis. Protein Eng. 1991 Feb;4(3):335–341. doi: 10.1093/protein/4.3.335. [DOI] [PubMed] [Google Scholar]
- McInnes C. J., Logan M., Redmond J., Entrican G., Baird G. D. The molecular cloning of the ovine gamma-interferon cDNA using the polymerase chain reaction. Nucleic Acids Res. 1990 Jul 11;18(13):4012–4012. doi: 10.1093/nar/18.13.4012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radford A. J., Hodgson A. L. Construction and characterization of a Mycobacterium-Escherichia coli shuttle vector. Plasmid. 1991 Mar;25(2):149–153. doi: 10.1016/0147-619x(91)90029-v. [DOI] [PubMed] [Google Scholar]
- Radford A. J., Hodgson A. L., Rothel J. S., Wood P. R. Cloning and sequencing of the ovine gamma-interferon gene. Aust Vet J. 1991 Mar;68(3):82–84. doi: 10.1111/j.1751-0813.1991.tb00755.x. [DOI] [PubMed] [Google Scholar]
- Rothel J. S., Jones S. L., Corner L. A., Cox J. C., Wood P. R. A sandwich enzyme immunoassay for bovine interferon-gamma and its use for the detection of tuberculosis in cattle. Aust Vet J. 1990 Apr;67(4):134–137. doi: 10.1111/j.1751-0813.1990.tb07730.x. [DOI] [PubMed] [Google Scholar]
- Smith D. B., Johnson K. S. Single-step purification of polypeptides expressed in Escherichia coli as fusions with glutathione S-transferase. Gene. 1988 Jul 15;67(1):31–40. doi: 10.1016/0378-1119(88)90005-4. [DOI] [PubMed] [Google Scholar]
- Smith P. K., Krohn R. I., Hermanson G. T., Mallia A. K., Gartner F. H., Provenzano M. D., Fujimoto E. K., Goeke N. M., Olson B. J., Klenk D. C. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985 Oct;150(1):76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- Wood P. R., Corner L. A., Plackett P. Development of a simple, rapid in vitro cellular assay for bovine tuberculosis based on the production of gamma interferon. Res Vet Sci. 1990 Jul;49(1):46–49. [PubMed] [Google Scholar]
- Wood P. R., Rothel J. S., McWaters P. G., Jones S. L. Production and characterization of monoclonal antibodies specific for bovine gamma-interferon. Vet Immunol Immunopathol. 1990 May;25(1):37–46. doi: 10.1016/0165-2427(90)90108-5. [DOI] [PubMed] [Google Scholar]