Abstract
Receptor activation of heterotrimeric G proteins dissociates Gα from the Gβγ complex, allowing both to regulate effectors. Little is known about the effector-interaction regions of Gβγ. We had used molecular modeling to dock a peptide encoding the region of residues 956–982 of adenylyl cyclase (AC) 2 onto Gβ to identify residues on Gβ that may interact with effectors. Based on predictions from the model, we synthesized peptides encoding sequences of residues 86–105 (Gβ86–105) and 115–135 (Gβ115–135) from Gβ. The Gβ86–105 peptide inhibited Gβγ stimulation of AC2 and blocked Gβγ inhibition of AC1 and by itself inhibited calmodulin-stimulated AC1, thus displaying partial agonist activity. Substitution of Met-101 with Asn in this peptide resulted in the loss of both the inhibitory and partial agonist activities. Most activities of the Gβ115–135 peptide were similar to those of Gβ86–105 but Gβ115–135 was less efficacious in blocking Gβγ inhibition of AC1. Substitution of Tyr-124 with Val in the Gβ115–135 peptide diminished all of its activities. These results identify the region encoded by amino acids 84–143 of Gβ as a surface that is involved in transmitting signals to effectors.
Heterotrimeric G proteins serve as signal transducers for a wide variety of receptors. Both Gα and Gβγ subunits can communicate receptor signals (1–5). Regions of Gβγ complex involved in communicating the signal to effectors have not been well characterized. We had identified the region of residues 956–982 of adenylyl cyclase (AC) 2 as being involved in receiving signals from Gβγ (6). By using the yeast two-hybrid system, the AC2 region of residues 956–982 has been subsequently shown to interact with Gβ but not Gγ subunits (7). In recent studies we found that the peptide encoding residues 956–982 of AC2 can be crosslinked to Gβ when it is part of the free Gβγ complex but not when it is part of the heterotrimer, indicating that the putative binding surface on Gβ for the AC2 peptide is occluded by interactions with Gα. On the basis of constraints deduced from the crosslinking studies and other biophysical criteria, we docked the AC2 peptide containing residues 956–982 onto the crystal structure of Gβ by using molecular modeling techniques (8). From this docking model, we have identified the regions of Gβ that are predicted to interact with the AC2 peptide. Herein we have tested whether peptides encoding the effector-interaction surface of Gβ predicted from the modeling (8) can modulate Gβγ regulation of AC1 and AC2.
MATERIALS AND METHODS
Materials.
Reagents for peptide synthesis were from Bachem. [α-32P]ATP was from New England Nuclear. Tissue culture reagents and fetal calf serum was from GIBCO. All other chemicals used were the highest grade available.
Peptide Synthesis.
Peptides were synthesized on an Applied Biosystems peptide synthesizer (model 431A) and purified by HPLC on acetonitrile gradients. Purified peptides were lyophilized and stored at −20°C. When required peptides were dissolved in water to a final concentration of 1–3 mM. Identity of the peptides was verified by mass spectrometry.
Expression of G-Protein Subunits and Adenylyl Cyclases.
Gβγ was purified from bovine brain (9). Q227L-Gαs was expressed in rabbit reticulocyte lysates. AC2 was expressed in Sf9 cells by infection with recombinant baculovirus (10). AC2 assays have been described (6). Bovine AC1 (11) was epitope tagged at the N terminus with the FLAG epitope (10) and expressed in Sf9 cells by baculovirus infection.
Adenylyl Cyclase Assays.
AC2 assays have been described (6). When required the peptides were mixed with adenylyl cyclase containing membranes and held on ice for 10 min prior to assays. Approximately 1–4 μg of AC2 Sf9 cell membranes per assay tube was used. All assays contained a mixture of protease inhibitors. Final concentration of the inhibitors were leupeptin at 3.2 μg/ml, aprotinin at 2 μg/ml, phenanthroline at 1.0 mM, and phenylmethylsulfonyl fluoride at 1.0 mM. To study Gβγ inhibition, AC1-containing Sf9 cell membranes (1–4 μg per assay tube) was used. In these assays, in addition to the other standard reagents, the assay mixture contained either 1 mM EGTA or 50 μM CaCl2 plus 100 nM calmodulin (CaM). All experiments were repeated two or more times with qualitatively similar results. Typical experiments are shown. Values are mean ± SD of triplicate determinations.
Molecular Modeling.
Procedures for molecular modeling have been described (8). Briefly, a secondary structure prediction of the AC2 peptide containing residues 956–982 (AC2 956–982) was obtained and used to construct an energy minimized three-dimensional model of the peptide. To identify likely interactions surfaces, the electrostatic potentials of the AC2 956–982 peptide and the Gβ protein (12) were visualized with the grasp program. Long-range electrostatic interactions were then used as guides in the initial docking of the peptide to Gβ. The structure of the AC2 956–982 peptide docked to Gβ was subjected to energy minimization followed by conformational explorations with a novel Monte Carlo-based method (13) The most favorable structure of the docked AC2 peptide interacting with Gβ was thus obtained within the imposed constraints. Contact residues on Gβ were identified with the look software (MAG, Palo Alto, CA) as residues within 4 Å of the AC2 peptide.
RESULTS
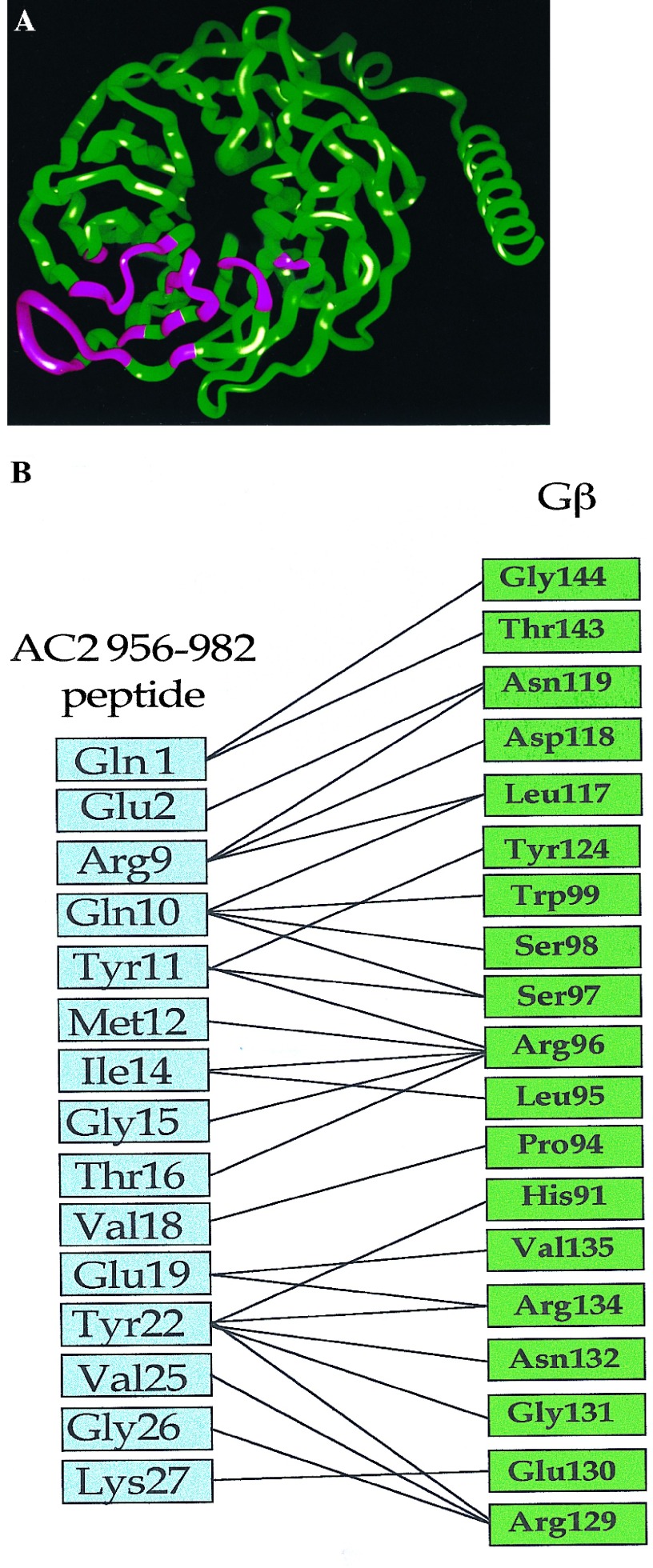
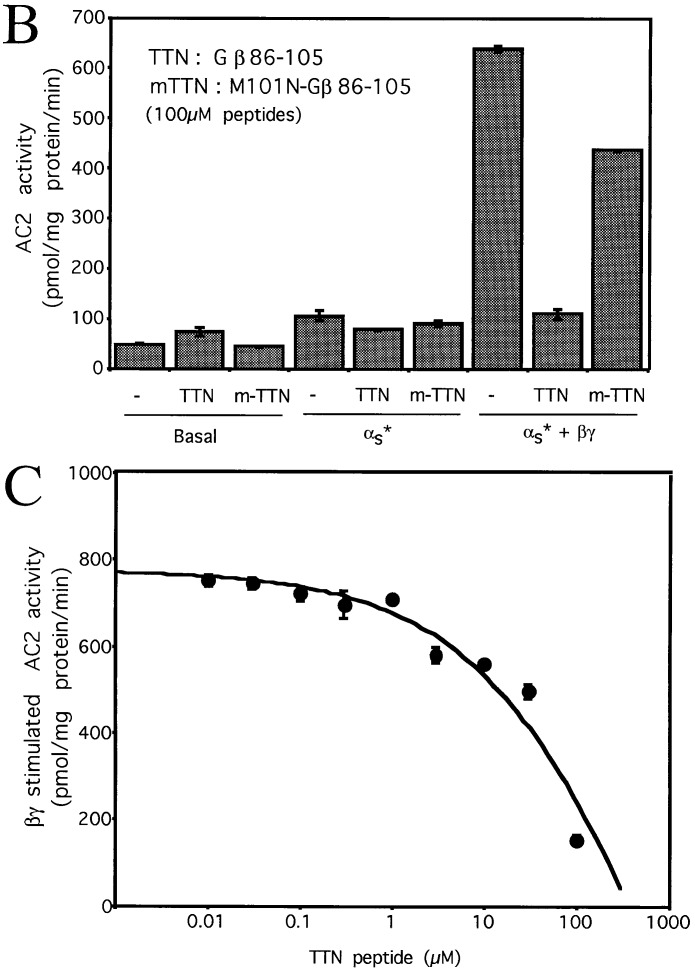
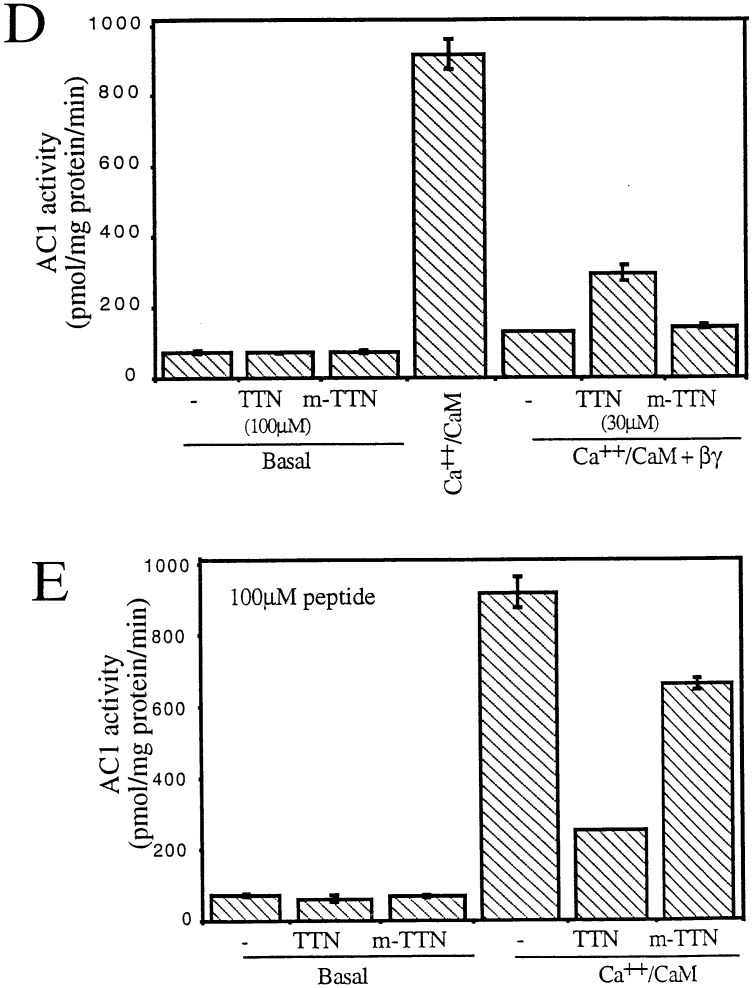
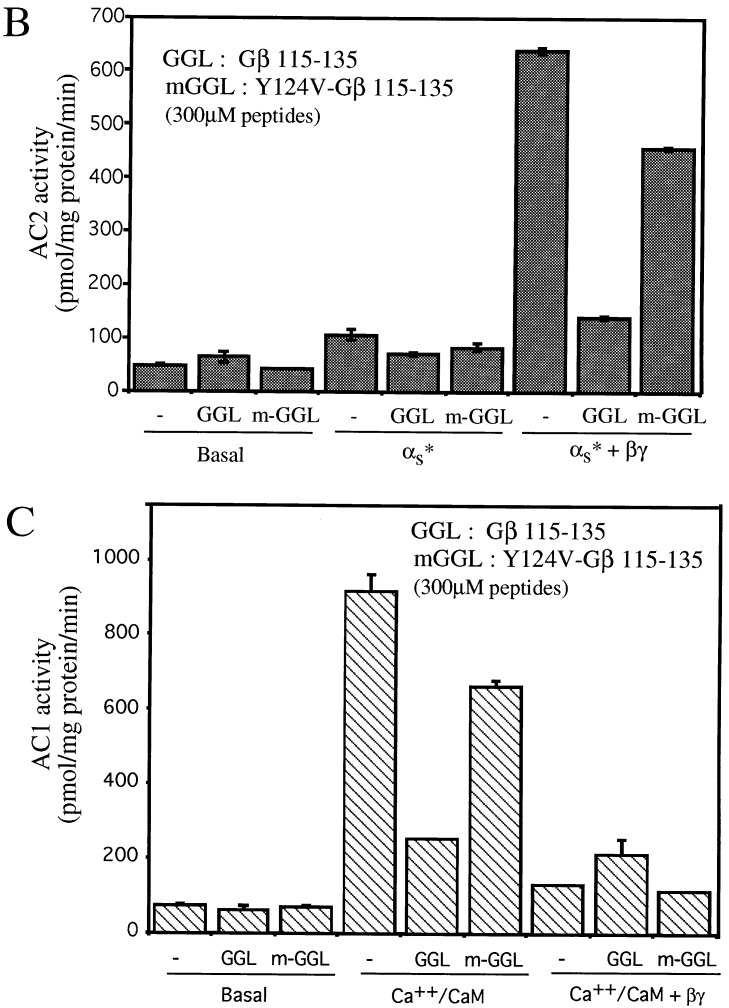
We used the docking model (8) to obtain predicted contact points between the Gβ and the AC2 956–982 peptide. Fig. 1A shows the backbone of Gβ. The regions of Gβ predicted to interact with the AC2 peptide are shown in pink. Predicted contacts between residues of the AC2 peptide and Gβ (see Materials and Methods) are shown in Fig. 1B. Since the peptide encodes a region of AC2, we reasoned that the predicted contact residues on Gβ could be involved in communicating signals to effectors. To test this idea, we synthesized peptides encoding sequences from Gβ and determined whether these peptides modulated Gβγ regulation of AC2 and AC1. Two peptides were designed based on the predicted contact interactions between Gβ and the AC2 peptide. The first peptide (TTN) encodes the region of residues 86–105 of Gβ, which includes the stretch of residues 91–99 predicted by our model to be important for effector interactions (Fig. 2A). The effects of TTN peptide on the activity of recombinant AC2 expressed in Sf9 cells are shown in Fig. 2. At 100 μM, the peptide did not inhibit basal or activated αs (αs*) stimulated activities; however, it significantly inhibited Gβγ-stimulated activity, which is seen only in the presence of αs* (1). To ascertain the specificity of the peptide effect, we substituted the residue corresponding to Met-101 in Gβ to Asn. This Met is conserved in most Gβ from different species (12) and mutation of the residue at this position in yeast abolishes Gα interactions (14). The “mutated” peptide (m-TTN) containing Asn at the position corresponding to Gβ-101 was much less efficacious than the TTN peptide in inhibiting Gβγ stimulation (Fig. 2B). The half-maximal concentration at which the TTN peptide inhibited Gβγ stimulation of AC2 was in the range of 30–60 μM (Fig. 2C). Since Gβγ also inhibits AC1, we tested whether the TTN peptide’s ability to block Gβγ interactions with effectors could be extended to modulation of Gβγ inhibition of AC1. The recombinant AC1 expressed in Sf9 cells was used in the assays. The TTN peptide did not affect basal activity of AC1. Gβγ inhibited the Ca2+/CaM-stimulated activity as expected. At 30 μM, the TTN peptide partially blocked Gβγ inhibition of Ca2+/CaM-stimulated AC1 activity whereas m-TTN did not affect Gβγ inhibition (Fig. 2D). Increasing concentrations of TTN peptide further did not result in greater blockade of Gβγ inhibition (data not shown). The reason for this became apparent when the effect of TTN peptide by itself was evaluated on the Ca2+/CaM-stimulated activity of AC1. The TTN peptide alone inhibited Ca2+/CaM activation of AC1 (Fig. 2E). At 100 μM, TTN peptide inhibited AC1 activity by 50–70%. The M101N “mutant” peptide had greatly reduced capacity to inhibit AC1 (Fig. 2E).
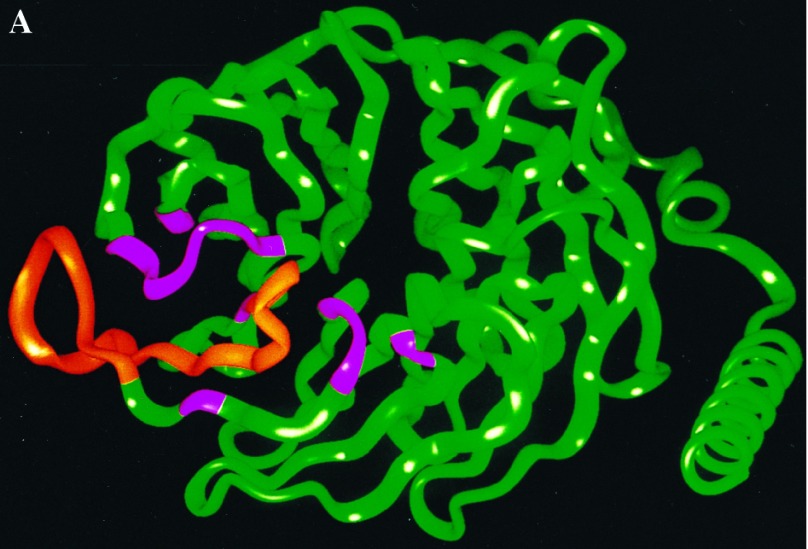
Figure 1.
Regions of Gβ involved in contacts with the AC2 956–982 peptide. (A) A ribbon diagram of the Gβ backbone from the crystal structure of Gβγ (12, 15); the residues in contact with the AC2 peptide are shown in pink (8). (B) Predicted core contacts between the AC2 956–982 peptide and Gβ. The AC2 peptide residues are in the blue boxes. The AC2 peptide residues are numbered 1–27 from the N terminus. Gβ1 residues are in green boxes. The Gβ1 residues are shown in the spatial sequence in which they are predicted to interact with the AC2 peptide.
Figure 2.
Effects of the Gβ86–105 peptides on AC2 and AC1 activities. (A) Ribbon diagram of the Gβ backbone with residues 86–105 in yellow. Other residues in contact with the AC2 peptide are shown in pink. (B) Effect of the Gβ86–105 peptide (TTN) and the M101N-Gβ86–105 mutant peptide (m-TTN) on basal, αs* (2 nM), and αs* (2 nM) plus Gβγ (50 nM) stimulated AC2 activities. (C) Effect of various concentrations of TTN peptide on Gβγ-stimulated AC2 activity in the presence of αs* (2 nM). (D) Effect of TTN and m-TTN peptides on basal and CaM (100 nM) plus Gβγ (30 nM) regulated AC1 activities. (E) Effect of TTN and m-TTN peptides on basal and CaM (100 nM) stimulated AC1 activities.
Two other regions of Gβ predicted by our model to be in contact with the crosslinked AC2 peptide are between residues 117–119 and 129–135 (Fig. 1B). Hence, we designed a second peptide (GGL) encoding the region of residues 115–135 of Gβ (Fig. 3A). The GGL peptide did not affect basal AC2 activity and did not significantly inhibit αs*-stimulated activity, but it did inhibit Gβγ stimulated activity (Fig. 3B). To assess the specificity of this peptide, we converted the residue corresponding to Tyr-124 in Gβ to a Val. This Tyr is conserved in all currently known Gβ from different species (12). This “mutated” peptide (m-GGL) was less effective in inhibiting Gβγ stimulation of AC2 (Fig. 3B). In contrast to its effect on AC2, the GGL peptide was not efficacious in blocking Gβγ-induced inhibition of AC1 (Fig. 3C). The m-GGL peptide also showed no effect on Gβγ inhibition of AC1 (Fig. 3C). Like the TTN peptide, the GGL peptide alone was also capable of inhibiting Ca2+/CaM-stimulated AC1 activity, but the m-GGL peptide did not inhibit the AC1 activity as extensively as the GGL peptide (Fig. 3C).
Figure 3.
Effects of the Gβ115–135 peptide on AC2 and AC1 activities. (A) Ribbon diagram of the Gβ1 backbone with residues 115–135 in yellow. Other residues in contact with the AC2 peptide are shown in pink. (B) Effect of the Gβ115–135 peptide (GGL) and the Y124V-Gβ115–135 mutant peptide (m-GGL) on basal, αs* (2 nM), and αs* (2 nM) plus Gβγ (50 nM) stimulated AC2 activities. (C) Effect of GGL and m-GGL peptides on basal, CaM (100 nM), or CaM (100 nM) plus Gβγ (30 nM) regulated AC1 activities.
DISCUSSION
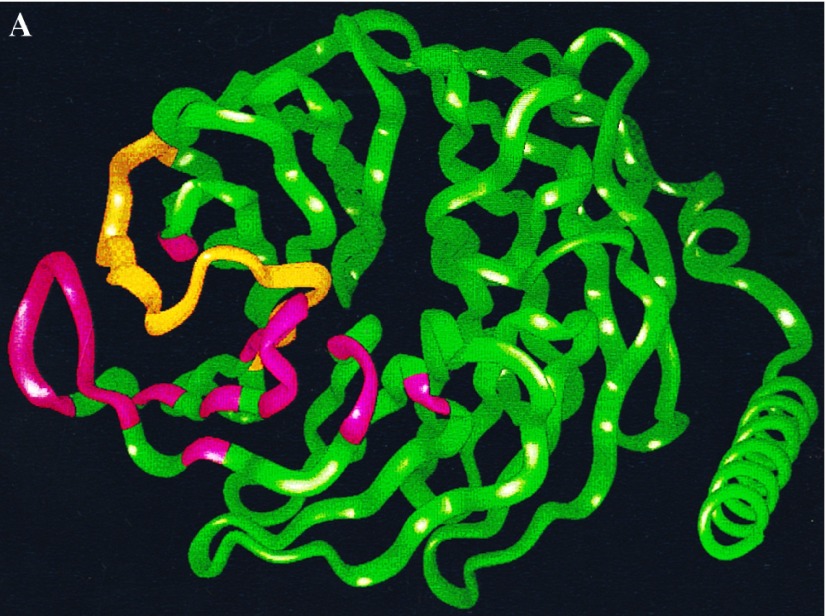
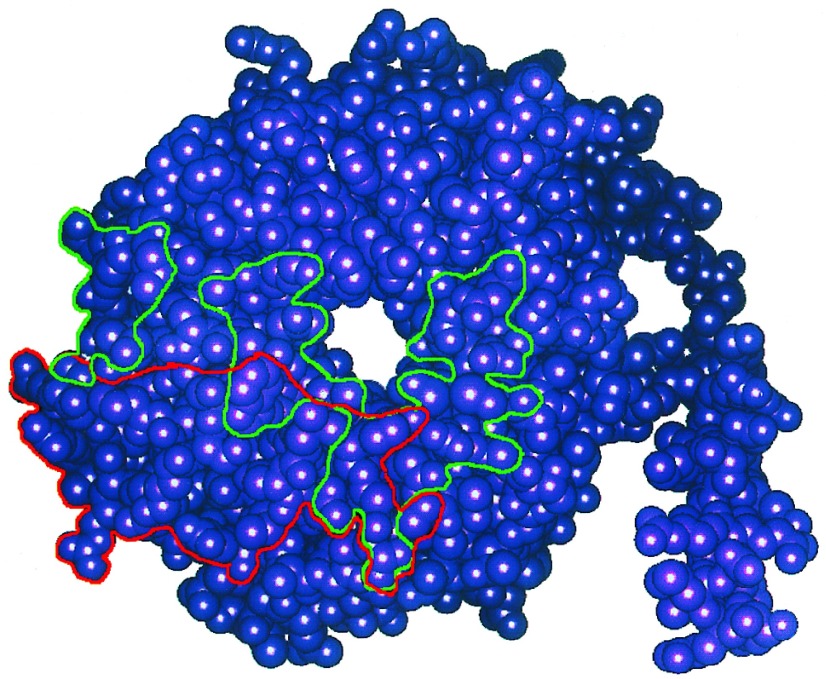
The results indicate that we have identified a surface on Gβ that is involved in effector interactions. The location of this region at the interface of Gα and Gβγ (8, 12) is consistent with the ability of Gα to block effector regulation by Gβγ, as many residues of Gβ that are involved in interactions with Gα, such as Trp-99, Met-101, Leu-117, and Asn-119 (12, 15), are also predicted by our model to interact with effectors. We have explicitly tested the importance of Met-101 that, as shown by the experiments in Fig. 2, is critical for regulation of effector function. We also showed that the conserved Tyr-124 of Gβ1 is important for effector regulation. Fig. 4 shows how the Gα binding region on Gβ identified from the crystal structure overlaps with an adenylyl cyclase (effector) interaction domain we have identified by molecular modeling. Further experiments are required to evaluate systematically the specific roles the many other residues we have identified in this effector interaction region.
Figure 4.
Schematic representation of the regions of Gβ involved in interactions with Gα (outlined in green) and some regions that may interact with adenylyl cyclases 1 and 2 (outlined in red). The space-filling model of Gβ was obtained from the crystallographic coordinates. Gα contact regions are those identified by Sigler and coworkers (12, 15) from the crystal structure of the heterotrimer. The AC2 peptide interaction region was deduced from molecular modeling studies (8) and the functional data in Figs. 2 and 3 indicate that these regions may be involved in interactions with AC1 and 2.
One issue that arises from these studies is whether the surface on Gβ where the AC2 peptide docks is sufficient for full effector contact. Our experiments indicate that the affinity provided by the interaction of the peptide from this surface is not sufficient to acheive full blockade of Gβγ stimulation of AC2 or to elicit full agonist activity of the Gβ peptides in regulating AC1. Interactions with additional regions of Gβ might be necessary. Alternatively, the remainder of the interactions required to achieve full contact with effectors could involve Gγ. Mutational analyses in yeast have identified three amino acid residues in the N-terminal part of Gγ that are required for effector function (16). The importance of the protein portion of Gγ in effector regulation remains to be investigated in biochemical experiments. It has also been shown that the posttranslational modification of Gγ that results in farnesylation (γ1 and possibly γ11) or geranylgeranylation (other γs) is required for effector interactions as assessed by biochemical assays with resolved components (17). These results suggest that the specific hydrophobic properties of the acyl group may be required for complete Gβγ action on effectors. Thus a more complete model for the mode of interaction of Gβγ with effectors may involve both the select protein regions in Gβ and the lipid moiety in Gγ.
Acknowledgments
This research was supported by National Institutes of Health Grants DK-38761 and GM-54508 to R.I. and K05DA00060 to H.W. and by funds from the Aaron Diamond Foundation. Y.C. is supported by a predoctoral fellowship from Molecular Endocrinology Training Grant DK-07645. G.W. is an Aaron Diamond Fellow. A.H. was supported by a predoctoral National Research Service Award (GM-15599). F.G. was supported by Grant 5T32DA07135. Computations were performed on the supercomputer systems at the Cornell National Supercomputer facility (sponsored by the National Science Foundation and IBM) as well as the Advanced Scientific Computing Laboratory at the Frederick Cancer Research Facility of the National Cancer Institute (Laboratory for Mathematical Biology).
ABBREVIATIONS
- AC
adenylyl cyclase
- CaM
calmodulin
References
- 1.Fung B K, Hurley J B, Stryer L. Proc Natl Acad Sci USA. 1981;78:152–156. doi: 10.1073/pnas.78.1.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Northup J K, Smigel M D, Sternweis P C, Gilman A G. J Biol Chem. 1983;258:11369–11376. [PubMed] [Google Scholar]
- 3.Logothetis D E, Kurachi Y, Galper J, Neer E J, Clapham D E. Nature (London) 1987;325:321–326. doi: 10.1038/325321a0. [DOI] [PubMed] [Google Scholar]
- 4.Tang W J, Gilman A G. Science. 1991;254:1500–1503. doi: 10.1126/science.1962211. [DOI] [PubMed] [Google Scholar]
- 5.Dietzel C, Kurjan J. Cell. 1987;50:1000–1010. doi: 10.1016/0092-8674(87)90166-8. [DOI] [PubMed] [Google Scholar]
- 6.Chen J, DeVivo M, Dingus J, Harry A, Li J, Sui J, Carty D J, Blank J L, Exton J H, Stoffel R H, Inglese J, Lefkowitz R J, Logothetis D E, Hildebrandt J D, Iyengar R. Science. 1995;268:1166–1169. doi: 10.1126/science.7761832. [DOI] [PubMed] [Google Scholar]
- 7.Yan K, Gautam N. J Biol Chem. 1996;271:17597–17601. doi: 10.1074/jbc.271.30.17597. [DOI] [PubMed] [Google Scholar]
- 8.Weng G, Li J, Dingus J, Hildebrandt J D, Weinstein H, Iyengar R. J Biol Chem. 1996;271:26445–26448. doi: 10.1074/jbc.271.43.26445. [DOI] [PubMed] [Google Scholar]
- 9.Dingus J, Wilcox M D, Kohnken R, Hildebrandt J D. Methods Enzymol. 1994;237:457–471. doi: 10.1016/s0076-6879(94)37083-4. [DOI] [PubMed] [Google Scholar]
- 10.Jacobowitz O, Iyengar R. Proc Natl Acad Sci USA. 1994;91:10630–10634. doi: 10.1073/pnas.91.22.10630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jacobowitz O, Chen J, Premont R T, Iyengar R. J Biol Chem. 1993;268:3829–3832. [PubMed] [Google Scholar]
- 12.Sondek J, Bohm A, Lambright D G, Hamm H E, Sigler P B. Nature (London) 1996;379:369–374. doi: 10.1038/379369a0. [DOI] [PubMed] [Google Scholar]
- 13.Guarnieri F, Weinstein H. J Am Chem Soc. 1996;118:5580–5589. [Google Scholar]
- 14.Whiteway M, Clark K L, Leberer E, Dignard D, Thomas D Y. Mol Cell Biol. 1994;14:3223–3229. doi: 10.1128/mcb.14.5.3223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lambright D G, Sondek J, Bohm A, Skiba N P, Hamm H E, Sigler P B. Nature (London) 1996;379:311–319. doi: 10.1038/379311a0. [DOI] [PubMed] [Google Scholar]
- 16.Grishin A V, Weiner J L, Blumer K J. Mol Cell Biol. 1994;14:4571–4578. doi: 10.1128/mcb.14.7.4571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Iniguez-Lluhi J A, Simon M I, Robishaw J D, Gilman A G. J Biol Chem. 1992;267:23409–23417. [PubMed] [Google Scholar]