Abstract
Abrupt termination of the treatment of humans with benzodiazepines (BDZs) leads to a rapid onset of discontinuation syndrome characterized by anxiety, muscle spasms, and occasionally convulsions. For this reason, it is recommended in clinical practice to reduce the dose of the BDZs gradually at the end of treatment. Nevertheless, many clinicians report signs of dependence even during gradual reduction of doses (tapering) of the BDZs in a large proportion of patients. Thus, there is considerable interest in discovering means of weaning patients away from BDZs without the risk of discontinuation syndrome. In the present study, mice withdrawn from chronic treatment with alprazolam showed anxiety, muscle rigidity, and seizures between days 1 and 28 after termination of the treatment. Replacement of alprazolam with the β-carboline abecarnil for 7 days prevented the occurrence of the signs of dependence. In contrast, substitution of the β-carboline antagonist ethyl-5-isopropoxy-4-methyl-β-carboline-3-carboxylate (ZK93426) for alprazolam worsened the discontinuation syndrome. Replacement therapy with abecarnil after long-term treatment with the BDZs offers a novel method for rapid tapering.
Keywords: benzodiazepines, withdrawal, tolerance, replacement therapy
Partial agonists at benzodiazepine (BDZ) receptors and compounds selective for subtypes of BDZ receptors possess advantages over full agonists in that, following long-term treatment, dependence is less (1, 2). The question arises whether such drugs may be useful in preventing the emergence of dependence on BDZs following long-term treatment, and whether subsequent termination of such treatment results in a discontinuation syndrome. Therefore, the purpose of the present study was to examine the time course of dependence following termination of long-term treatment with the short half-life BDZ alprazolam, and to study the ability of the subtype selective β-carboline BDZ receptor agonist abecarnil (3, 4) to prevent discontinuation syndrome in mice dependent on alprazolam.
Alprazolam, a BDZ with a short half-life, was chosen because it recently has replaced diazepam as the most frequently prescribed anxiolytic drug (5). This shift in the pattern of drug prescription was justified by the low likelihood of alprazolam accumulation (compared with diazepam) and causing sedative effects on multiple doses (5). Nevertheless, a major drawback of long-term treatment with alprazolam is that sustained effects could be achieved only on multiple daily dosing regimens and that discontinuation of such therapy was most likely to induce signs of dependence (6). Indeed, it is commonly held that BDZs with short half-lives have greater dependence liability than compounds with longer half-lives, although this view is not adequately supported by controlled human data (5). There are also no adequately controlled animal experimental data that allow comparison of the risk of producing dependence between BDZs with short and long half-lives (7). Similarly, there is scanty experimental data demonstrating how to discontinue safely treatment with BDZs with short half-lives.
To address this issue we have employed newly developed methods for electroencephalographic (EEG) monitoring of seizures, for electromyographic (EMG) monitoring of muscle tone, and for detecting anxiety-like behavioral changes after discontinuation of long-term treatment with sedative drugs in mice (8) for controlled and standardized assessment of dependence liability of alprazolam. Using these experimental approaches, we describe the time course of the discontinuation syndrome after termination of long-term treatment with alprazolam in mice, and propose a therapeutic strategy for preventing signs of withdrawal using substitution with the β-carboline abecarnil.
MATERIALS AND METHODS
Animals.
Male NMRI mice (Schering), 20–24 g in weight, were subjected to s.c. injections of 6 mg/kg of alprazolam (8-chloro-1-methyl-6-phenyl-4H-[1,2,4]triazolo[4,3-a][1,4]benzodiazepine; Upjohn) or vehicle (sesame oil) given twice daily (9:00 a.m. and 9:00 p.m.) for 12 consecutive days. Alprazolam was dissolved in sesame oil and administered in a volume of 0.04 ml/10 g body weight. Such treatment led to full development of tolerance to the sedative action of alprazolam (see Fig. 1). Monitoring of withdrawal signs started on the day following the last administration of alprazolam or vehicle. This day was designated withdrawal day 1 (W1). After withdrawal all experimental animals received daily injections of vehicle.
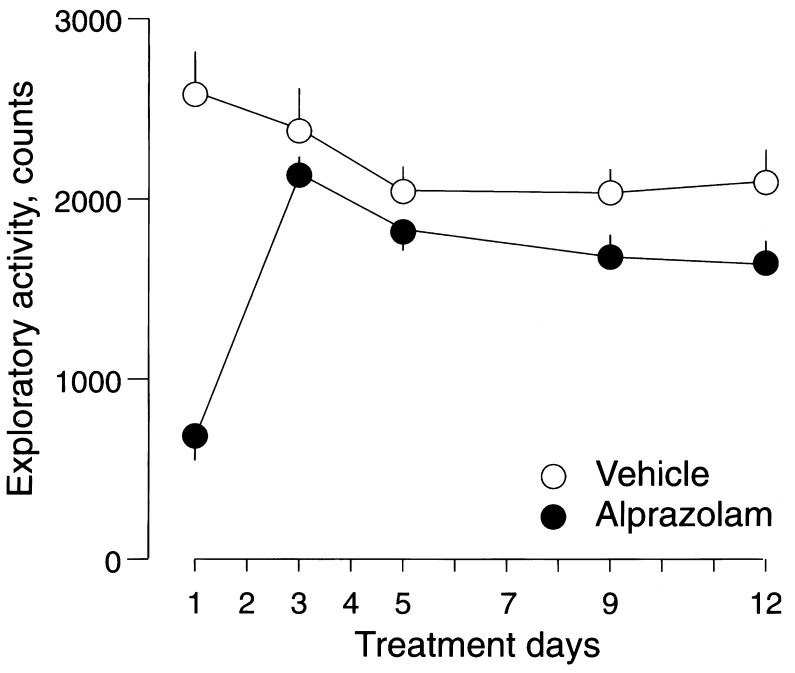
Figure 1.
Time course of changes in the exploratory activity of mice during chronic treatment with alprazolam. Circles represent mean ± SEM number of counts recorded in mice treated with vehicle (○) or alprazolam at 6 mg/kg twice daily (•). Exploratory activity was monitored for 10 min in nonhabituated mice 2 h after s.c. administration of vehicle or alprazolam. Two-way ANOVA with factors for treatment, time, and their interaction revealed a rapid development of tolerance to the sedative effect of alprazolam during chronic treatment, FT1(1,136) = 47.33, P < 0.001 vs. FT3-T12(1,136) = 1.25, P > 0.05). Experimental groups consisted of 16 mice, which were used for the monitoring of exploratory activity once only during the entire treatment with vehicle or alprazolam over 12 days. A total of 146 recordings performed in 160 mice were used for the analysis.
Monitoring of Withdrawal Syndromes in Mice.
Electroencephalography. For long-term EEG monitoring [Grass polygraph (Grass Instruments, Quincy, MA); time constant, 0.03 s; high cut-off filter, 15 Hz], mice were stereotaxically implanted with bipolar twisted electrodes (tip diameter, 100 μm; interelectrode distance, 500 μm) positioned in the dorsal hippocampus (AP 2.5; L 2.0; V 3.5) (9) under sodium pentobarbital anesthesia [Nembutal; Ceva (Neuilly-sur-Seine, France), 50 mg/kg i.p.]. Surface recordings were led from screws positioned bilaterally over the occipital cortex. The surgery was performed 3–5 days before the first administration of alprazolam or vehicle. EEG recordings started on W1 at 9:00 a.m. and were carried out in circular Perspex cages (diameter 40 cm, height 40 cm) located in a sound-insulated room. Mice were chronically treated with alprazolam (6 mg/kg s.c. twice daily) or vehicle for 12 days. The monitoring was continued for up to 28 days after the last alprazolam or vehicle administration and was discontinued for periods of 10–15 min/day (between 8:30–9:00 a.m.) for daily care. Video and EEG signals were stored on computer discs and magnetic tape. Videotaping during the nocturnal phase was made using infrared cameras. Seizure recognition was performed on-line using an extended Compaq computer system (Biomedical Monitoring Systems, Campbell, CA) equipped with a detection program (monitor 5.1; Stellate Systems; Montreal) (8). Computer-identified seizure patterns were reanalyzed off-line by an observer unaware of drug treatment, and artifacts were discarded. The correct location of the implanted deep electrodes was histologically controlled in cresyl violet-stained serial sections of the entire brain 5–7 days after the last observation session.
Electromyography.
The spontaneous activity in the EMG was recorded from the gastrocnemius muscle of the mice using pairs of Teflon-insulated stainless-steel wire electrodes (Cooner Wire, Chatsworth, CA; AS 632 SS) inserted percutaneously into the muscle (10). The mice were placed separately in ventilated Perspex boxes and their hindlimbs, gently secured with adhesive tape, were extended through slots in the bottom of the boxes. The electrical signals were amplified, band-pass filtered (5–10 kHz), full-wave rectified, and stored on discs and paper. The EMG was recorded continuously, and the average integrated activity was determined for 1 h. To ensure that only tonic EMG activity was measured, bursts of phasic activity in EMG lasting less than 30 s due to movements of the mouse were discarded. Recordings were performed between 9:00 and 11:00 a.m.
Locomotor activity and movement tracking.
A computerized Digiscan-16 Animal Activity Monitoring System (Omnitech Electronics, Columbus, OH) was used to quantify and track locomotor activity in mice. Each activity monitor consisted of a Perspex box (40 × 40 × 40 cm) surrounded by 48 horizontal and vertical infrared sensor beams. The total number of interruptions of the horizontal sensors was taken as a measure of horizontal activity, while that of vertical sensors as a measure of vertical activity (11). Furthermore, the time spent by the mice moving in close proximity to the walls (closer than 1 cm) or in the center (>1 cm) was recorded. Locomotor activity monitoring and movement tracking was performed in independent groups of nonhabituated mice for 10 min between 9:00 and 11:00 a.m. on each experimental day. Mice showing a pattern of activity limited to movements along the cage walls or in one cage compartment (i.e., avoiding the center of the cage) were designated “anxious.” Therefore, the margin time/center time ratio was used as a measure of anxiety. Similar patterns of locomotor activity were induced in mice by drugs such as picrotoxin, pentylenetetrazol, and ethyl β-carboline-3-carboxylate (8), reported to give rise to anxiety-like effects in other models.
Abecarnil/ethyl-5-isopropoxy-4-methyl-β-carboline-3-carboxylate (ZK93426) and alprazolam dependence.
Mice withdrawn from long-term treatment (12 days) with alprazolam or vehicle were subjected to seven daily (9 a.m.) s.c. injections of either 6 mg/kg of abecarnil (isopropyl-6-benzyloxy-4-methoxymethyl-β-carboline-3-carboxylate; Schering), 20 mg/kg of ZK93426 (Schering), 6 mg/kg alprazolam twice daily (9 a.m. and 9 p.m.), or vehicle (sesame oil). Abecarnil, ZK93426, and alprazolam were dissolved in sesame oil and administered in a volume of 0.04 ml/10 g body weight. Monitoring of withdrawal signs started on the day following the last administration of alprazolam and was continued during replacement treatment with abecarnil, ZK93426, alprazolam, or vehicle for 7 days, and the following 21 days after discontinuation of the replacement treatment. The day following the last administration of alprazolam was designated replacement day 1. The day following the last administration of substituting agents (abecarnil, ZK93426, alprazolam, or vehicle) was designated W1. During withdrawal all experimental animals received daily injections of vehicle. The treatment regimen chosen for abecarnil and ZK93426 was selected in such a way that the doses used were the highest not inducing sedation or performance disturbances of mice on the rota-rod as measured daily during the entire period of chronic administration.
Statistics.
The experimental data were analyzed statistically by means of two-way ANOVA, repeated measures ANOVA, and multivariate ANOVA.
RESULTS
Tolerance to Alprazolam.
Long-term treatment with alprazolam led to a rapid loss of its depressant action on exploratory activity in nonhabituated mice measured as the number of interruptions of horizontal sensors (Fig. 1).
Withdrawal.
Mice withdrawn from long-term (12 days) treatment with alprazolam showed electrographic seizures, increase in EMG activity, and a pattern of exploratory activity reminiscent of anxiety (Fig. 2). In animals withdrawn from long-term treatment with vehicle, no seizures were detected in EEG, no changes in muscle tone were registered in EMG, and no change in the pattern of exploratory activity measured as the margin time/center time ratio was observed (Fig. 2).
Figure 2.
Time course of seizures, changes in muscle tone, and anxiety in mice subjected to chronic treatment with alprazolam or vehicle on withdrawal. Circles represent mean ± SEM number of seizures per animal recorded in groups of 4 vehicle- and 9 alprazolam-treated mice (A), mean ± SEM EMG activity in μV/s recorded in groups of 10 vehicle- and 10 alprazolam-treated mice (B), and mean ± SEM anxiety expressed as margin time/center time ratio from groups of 10 vehicle- and 13 alprazolam-treated mice (C), withdrawn from vehicle (○), or alprazolam (•). Multivariate ANOVA on the treatments in EMG, using the 16 measurement days as variables, showed a significant difference in the overall effect (FEMG(16,2) = 13313.54, P < 0.001), and two-way ANOVA with factors for treatment, time, and their interaction on anxiety expressed as margin time/center time ratio (Mtime/Ctime) demonstrated significant effect of treatment (FMtime/Ctime(1,276) = 256.89, P < 0.001), revealing that chronic treatment with alprazolam resulted in seizures, muscle rigidity, and anxiety on withdrawal. A total of 320 recordings performed in 20 mice were used for the analysis of the EMG, while 308 recordings from 368 mice were employed for the analysis of anxiety.
Seizures.
The EMG seizures were most frequent between W4 and W14 (Fig. 2A). Fewer seizures were detected during the first 3 days and after 21 withdrawal days. The duration of seizures rarely exceeded 60 s. The majority of the seizures were first seen in the hippocampus and then spread rapidly to cortical recordings. In terms of behavior, the seizures were characterized by initial akinesia, automatisms, forelimb clonus, rearing, and falling. No seizures were detected during the entire observation period (up to 28 days) following cessation of the treatment of mice with vehicle (Fig. 2A).
EMG Activity.
EMG monitoring showed increased activity in the gastrocnemius muscle in mice withdrawn from alprazolam (Fig. 2B). EMG activity was maximal between W3 and W7. Little or no muscle rigidity was registered between W8 and W28. Abrupt termination of the treatment of mice with vehicle did not result in changes in the muscle tone during the entire period of monitoring (Fig. 2B).
Anxiety.
In mice withdrawn from alprazolam, anxiety was maximal between W1 and W12 (Fig. 2C). Gradually decreasing anxiety was recorded during W14–W28 (Fig. 2C). A decrease in the margin time/center time ratio indicated that the animals spent more time moving at the cage walls, reflecting the changes in locomotor activity patterns in mice withdrawn from alprazolam (Fig. 2C). No difference between alprazolam- and vehicle-withdrawn mice was detected over the entire observation period in exploratory activity measured as horizontal activity (counts) or total distance (cm). No change in the margin time/center time ratio was detected in mice withdrawn from vehicle (Fig. 2C).
Effects of Abecarnil and ZK93426 on Alprazolam Dependence.
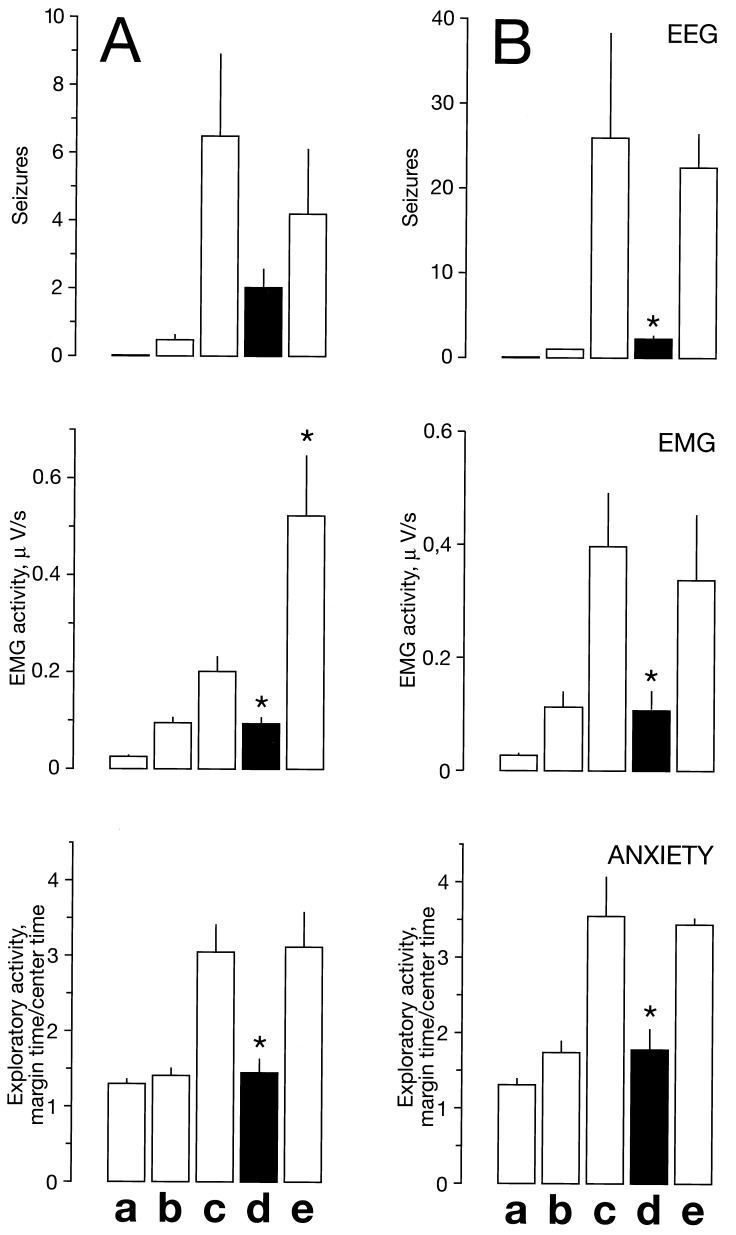
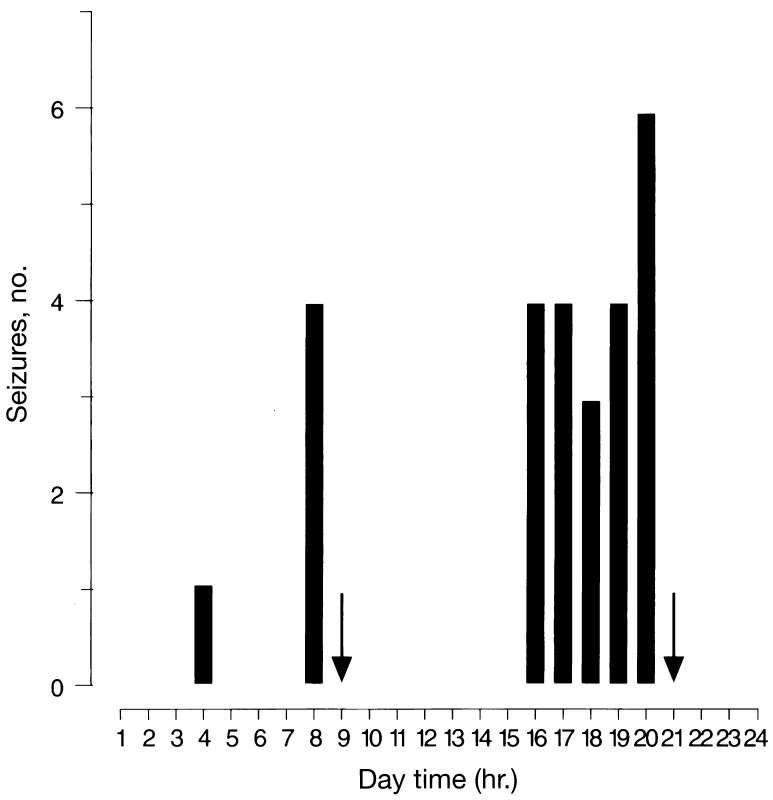
Treatment with abecarnil during the first 7 days of alprazolam withdrawal prevented an increase in the EMG activity and anxiety and reduced electrographic seizures (Fig. 3A). In contrast, 7 days of treatment with ZK93426 did not affect alprazolam withdrawal in measures of electrographic seizures and anxiety, but did increase rigidity (Fig. 3A). When long-term treatment with alprazolam was continued for the following 7 days, the withdrawal was not affected (Figs. 3B and 4). Surprisingly, withdrawal seizures were already recorded during alprazolam treatment and were most frequent shortly before each administration of the BDZ (Fig. 4). Treatment of mice with vehicle during the first 7 days after discontinuation of alprazolam administration did not affect the intensity of withdrawal as monitored by EEG and EMG, and observed in locomotor activity patterns (Fig. 3B).
Figure 3.
Effect of abecarnil, ZK93426, alprazolam, or vehicle on seizures, changes in muscle tone, and anxiety in alprazolam-dependent mice during the replacement treatment (7 days; A) and on withdrawal after the replacement treatment (21 days; B). Bars represent mean ± SEM number of seizures per animal, mean ± SEM EMG activity in μV/s, and mean ± SEM anxiety expressed as margin time/center time ratio in exploratory activity experiments. Groups of 4–16 mice subjected to chronic treatment (12 days) with 6 mg/kg of alprazolam (twice daily) or vehicle were injected s.c. with either 6 mg/kg of abecarnil, 20 mg/kg of ZK93426, 6 mg/kg of alprazolam (twice daily), or vehicle for 7 days. Bars: a, vehicle; b, vehicle plus abecarnil; c, alprazolam plus alprazolam; d, alprazolam plus abecarnil; e, alprazolam plus ZK93426. ∗, P < 0.05 vs. alprazolam plus alprazolam-treated mice (ANOVA).
Figure 4.
Histogram of the distribution of spontaneous recurrent seizures during the day (6:00 a.m. to 6:00 p.m. light/dark cycle) detected in the course of replacement treatment with alprazolam (7 days) in four mice previously subjected to 12 days of alprazolam (6 mg/kg s.c., twice daily) treatment. Arrows indicate the injection of alprazolam. The number of seizures was calculated for 1-h epochs over 7 days.
DISCUSSION
Termination of long-term treatment with alprazolam leads to the occurrence of signs of dependence in measures of electrographic seizures and changes in muscle tone and anxiety. The intensity of the discontinuation syndrome increases only slightly over the first 2–3 days of withdrawal, the symptoms were most pronounced during the next 7–14 days, and abated slowly up to W21–W28. Long-term electrophysiological monitoring over 7 days preceding discontinuation of alprazolam treatment showed that signs of dependence already appear during full-dose treatment with the BDZ and well before termination of the therapy. Replacement of alprazolam treatment with the β-carboline selective agonist abecarnil prevented the occurrence of withdrawal signs; when replacement treatment with abecarnil was subsequently terminated no signs of dependence were detected. Replacement of alprazolam treatment with the β-carboline antagonist ZK93426 did not prevent the discontinuation syndrome.
Substitution of abecarnil for alprazolam blocked the occurrence of signs of dependence. The observation that abecarnil was more effective than a continued treatment with alprazolam in this respect is probably attributable to the kinetics of abecarnil administered in a depot formulation, since we have previously described high receptor occupancies by abecarnil throughout the 24-h period following a single daily administration, which would be expected to prevent “breakthrough” withdrawal events seen under alprazolam treatment using the same formulation (12).
The rapid onset of the discontinuation syndrome is known to occur in patients who have taken BDZs with short half-lives, whereas patients taking BDZs with long half-lives develop the discontinuation syndrome with a delay of 3–8 days, reflecting the different elimination kinetics (6). Using long-term electrophysiological monitoring in mice withdrawn from diazepam, we demonstrated that the discontinuation syndrome occurs after a delay of about 3 days (8). Using similar experimental approaches, we now report that the discontinuation syndrome following the termination of a chronic treatment with alprazolam occurs the day after the discontinuation of the drug. These similarities between clinical and experimental experience indicate the relevance of the experimental approaches employed in our studies.
The long-term use of the BDZs for therapy of sleep disturbances and anxiety has become a cause of scientific and public concern because of the danger of physical dependence. Such concerns provoked the design of new generation anxiolytic drugs supposed to have no (or less than BDZs) dependence liability. Abecarnil, a β-carboline that acts at BDZ receptors, markedly reduces dependence potential compared with BDZs in rodents, dogs, cats, and in non-human primates (12–15). Although abecarnil acts as a high-affinity full agonist at BDZ receptors containing α1 subunits, it may act as a partial agonist at other receptor subtypes (16). Surprisingly, in mice, replacement of alprazolam treatment with abecarnil (for 1 week) reduced the severity of signs of alprazolam withdrawal during the course of treatment with abecarnil, and prevented the emergence of the alprazolam discontinuation syndrome after abrupt termination of the therapy with abecarnil.
The dosing regimen for alprazolam employed in this study was chosen such that the treatment was expected to approximate the kinetics of alprazolam in the clinical situation in the majority of the patients undergoing long-term therapy. In those patients, signs of dependence are frequently reported to occur during the course of ongoing treatment with alprazolam (5). Similarly, in mice undergoing long-term treatment with alprazolam, signs of dependence were observed in the course of the medication. The therapeutic success of abecarnil in preventing the emergence of dependence signs after discontinuation of the long-term treatment with alprazolam may be explained by its long half-life compared with alprazolam—i.e., it may well be that in the experimental situation chosen, replacement of alprazolam by abecarnil led to better relative coverage of the BDZ receptors. Surprisingly, subsequent withdrawal from abecarnil did not lead to the occurrence of signs of dependence, suggesting that during the replacement of alprazolam with abecarnil in dependent mice, changes had occurred in mechanisms responsible for the expression of the discontinuation syndrome. The major question is how this adaptation could occur during the presence of a drug sufficient to prevent the expression of alprazolam-withdrawal signs. One possibility might be that, because abecarnil acts as a full agonist at a portion of the BDZ receptors, it is able to prevent the emergence of signs of dependence. Concurrently, its action as a partial agonist at other BDZ receptors may allow them to re-adapt to the alprazolam-free state (17). Such an explanation presupposes that both those receptors at which abecarnil acts as a full agonist, and those at which it acts as a partial agonist, are involved in the mechanism of BDZs to suppress epileptiform events, enhanced muscle tone, and anxiety-like behavior. Whatever the ultimate explanation for our findings, if such observations can be confirmed in the clinical practice, the challenge with abecarnil could allow abrupt termination of long-term treatment with BDZs.
There are anecdotal reports from the psychiatric clinic that the BDZ antagonists may be beneficial in some patients for preventing dependence following long-term treatment with BDZs. We therefore addressed the question whether replacement therapy (for 1 week) with the β-carboline ZK93426 might improve the clinical outcome in mice withdrawn from long-term treatment with alprazolam. ZK93426 is a broad spectrum BDZ antagonist and possesses a short half-life (18). ZK93426 increased the severity of the signs of dependence in the course of its administration as measured by an increase in the muscle tone, and lacked a beneficial effect on the frequency of seizures or the expression of anxiety-like behavior. Termination of the treatment with ZK93426 did not affect the severity of the discontinuation syndrome monitored in mice withdrawn from alprazolam. These observations do not support the notion that antagonism at BDZ receptors may prevent or reverse BDZ dependence.
The relevance of our studies lies in the controlled and standardized evaluation of dependence liability of alprazolam in mice. Termination of long-term treatment with alprazolam led to the rapid expression of the discontinuation syndrome. Several aspects of dependence could be already seen in animals undergoing long-term treatment with alprazolam, indicating the dosing regimen does not allow covering all the BDZ receptors during the ongoing medication. The β-carboline agonist at BDZ receptors abecarnil which possesses reduced dependence potential may be of value for preventing the signs of dependence in humans withdrawn from BDZs. Replacement therapy with abecarnil after long-term treatment with the BDZs offers a novel method for rapid tapering. The use of the BDZ antagonists for tapering long-term treatment with BDZs is not justified by the experimental data.
ABBREVIATIONS
- BDZ
benzodiazepine
- ZK93426
ethyl-5-isopropoxy-4-methyl-β-carboline-3-carboxylate
- EEG
electroencephalogram/electroencephalographic
- EMG
electromyogram/electromyographic
- W
withdrawal day(s)
References
- 1.Haefely W, Martin J R, Schoch P. Trends Pharmacol Sci. 1990;11:452–456. doi: 10.1016/0165-6147(90)90126-s. [DOI] [PubMed] [Google Scholar]
- 2.Haefely W, Facklam M, Schoch P, Martin J R, Bonetti E P, Moreau J-L, Jenck F, Richards J G. In: GABAergic Synaptic Transmission. Biggio G, Concas A, Costa E, editors. New York: Raven; 1992. pp. 379–394. [Google Scholar]
- 3.Stephens D N, Schneider H H, Kehr W, Andrews J S, Rettig K-J, Turski L, Schmiechen R, Turner J D, Jensen L H, Petersen E N, Honore T, Bondo Hansen J. J Pharmacol Exp Ther. 1990;253:334–343. [PubMed] [Google Scholar]
- 4.Turski L, Stephens D N, Jensen L H, Petersen E N, Meldrum B S, Patel S, Bondo Hansen J, Löscher W, Schneider H H, Schmiechen R. J Pharmacol Exp Ther. 1990;253:344–352. [PubMed] [Google Scholar]
- 5.Shader R I, Greenblatt D J. N Engl J Med. 1993;328:1398–1405. doi: 10.1056/NEJM199305133281907. [DOI] [PubMed] [Google Scholar]
- 6.Busto U, Sellers E M, Naranjo C A, Cappell H, Sanchez-Craig M, Sykora K. N Engl J Med. 1986;315:845–849. doi: 10.1056/NEJM198610023151403. [DOI] [PubMed] [Google Scholar]
- 7.Woods J H, Katz J L, Winger G. J Am Med Assoc. 1988;260:3476–3480. [PubMed] [Google Scholar]
- 8.Steppuhn K G, Turski L. Proc Natl Acad Sci USA. 1993;90:6889–6893. doi: 10.1073/pnas.90.14.6889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Montemurro D G, Dukelow R H. A Stereotaxic Atlas of the Diencephalon and Related Structures of the Mouse. Mount Kisco, NY: Futura; 1972. [Google Scholar]
- 10.Turski L, Klockgether T, Schwarz M, Turski W A, Sontag K-H. Ann Neurol. 1990;28:341–348. doi: 10.1002/ana.410280307. [DOI] [PubMed] [Google Scholar]
- 11.Klockgether T, Turski L. Ann Neurol. 1990;28:539–546. doi: 10.1002/ana.410280411. [DOI] [PubMed] [Google Scholar]
- 12.Steppuhn K G, Schneider H H, Turski L, Stephens D N. J Pharmacol Exp Ther. 1993;264:1395–1400. [PubMed] [Google Scholar]
- 13.Löscher W. In: Anxiolytic β-Carbolines: From Molecular Biology to the Clinic. Stephens D N, editor. Berlin: Springer; 1993. pp. 96–112. [Google Scholar]
- 14.Serra M, Ghiani C A, Motzo C, Biggio G. In: Anxiolytic β-Carbolines: From Molecular Biology to the Clinic. Stephens D N, editor. Berlin: Springer; 1993. pp. 62–78. [Google Scholar]
- 15.Sannerud C A, Ator N A, Griffith R R. Behav Pharmacol. 1992;3:507–516. [PubMed] [Google Scholar]
- 16.Pribilla I, Neuhaus R, Hillmann M, Turner J D, Stephens D N, Schneider H H. In: Anxiolytic β-Carbolines: From Molecular Biology to the Clinic. Stephens D N, editor. Berlin: Springer; 1993. pp. 50–61. [Google Scholar]
- 17.Stephens D N, Turski L. In: Benzodiazepine Receptor Inverse Agonists. Nutt D J, Sarter M, editors. New York: Wiley–Liss; 1995. pp. 83–112. [Google Scholar]
- 18.Jensen L H, Petersen E N, Braestrup C, Honore T, Kehr W, Seidelmann D, Schmiechen R. Psychopharmacology. 1984;83:249–256. doi: 10.1007/BF00464789. [DOI] [PubMed] [Google Scholar]