Abstract
Previous studies have shown that chronic administration of class I antiarrhythmic drugs, which have definite inhibitory action on the fast Na+ channel, result in up-regulation of cardiac Na+ channel expression, and suggest that this effect may contribute to their deleterious effects during chronic administration. Recent studies have shown that the antiarrhythmic effects of free n − 3 polyunsaturated fatty acids (PUFA) are associated with an inhibition of the Na+ channel. Whether the PUFA when used chronically will mimic the effect of the class I drugs on the expression of the Na+ channel is not known. To answer this question, we determined the level of mRNA encoding cardiac Na+ channels and the number of the Na+ channels per cell in cultured neonatal rat cardiac myocytes after supplementation of the cells with the n − 3 PUFA eicosapentaenoic acid (EPA), the class I drug mexiletine, or both EPA and mexiletine for 3–4 days. The number of sodium channels was assessed with a radioligand binding assay using the sodium channel-specific toxin [3H]batrachotoxinin benzoate ([3H]BTXB). The supplementation of myocytes with mexiletine (20 μM) induced a 4-fold increase in [3H]BTXB specific binding to the cells. In contrast, chronic treatment with EPA (20 μM) alone did not significantly affect [3H]BTXB binding. However, the combination of EPA with mexiletine produced a 40–50% reduction in the [3H]BTXB binding, compared with that seen with mexiletine alone. RNA isolated from cardiac myocytes was probed with a 2.5-kb cRNA transcribed with T7 RNA polymerase from the clone Na-8.4, which encodes nucleotides 3361–5868 of the α-subunit of the RIIA sodium channel subtype. The changes in the level of mRNA encoding sodium channel α-subunit were correlated with comparable changes in sodium channel number in the cultured myocytes, indicating that regulation of transcription of mRNA or its processing and stability is primarily responsible for the regulation of sodium channel number. These data demonstrate that chronic EPA treatment not only does not up-regulate the cardiac sodium channel expression but also reduces the mexiletine-induced increase in the cardiac sodium channel expression.
According to the Cardiac Arrhythmia Suppression Trial (1), the commonly used class I antiarrhythmic drugs that act by inhibiting cardiac Na+ channels are not safe or effective, since chronic treatment with the drugs (encainide or flecainide) produced a poorer outcome with a higher mortality than placebo despite their suppression of premature ventricular complexes with short term use. Although the mechanisms responsible for these deleterious effects during chronic administration of the class I antiarrhythmic drugs are not fully understood, one possible explanation comes from the findings reported from the laboratories of Catterall and Duff (2, 3). They found that chronic treatment in rats with the class I antiarrhythmic drug mexiletine resulted in upregulation of cardiac Na+ channel expression, as shown by increase in both the level of mRNA encoding sodium channel α-subunits and the number of sodium channels per cell. It was proposed that the increased number of sodium channels caused by chronic treatment with these drugs may itself cause arrhythmias as a secondary consequence of therapy (2, 3). These previous observations indicate the importance of careful reevaluation of the safety of other forms of antiarrhythmic agents and the need of development of a safe and highly effective means of preventing lethal arrhythmias.
Recent studies have shown a role for n − 3 polyunsaturated fatty acids (PUFA) in the prevention of fatal ventricular arrhythmias (for review, see ref. 4). We have found that the antiarrhythmic effects of the fatty acids are associated with their inhibitory action on cardiac excitability/automaticity, an important factor influencing the generation and the termination of arrhythmias (5–8). Our electrophysiological study shows that free PUFA significantly increase the threshold for gating the fast Na+ channel (which initiates the action potential), hyperpolarizes the resting or diastolic membrane potential, and prolongs the refractory period duration in rat cardiac myocytes (8). The increase in threshold for the gating of the fast Na+ channel indicates that Na+ currents through this channel are modulated by the PUFA. This has been confirmed by our recent study showing that n − 3 PUFA inhibited Na+ currents in a dose-, time-, and voltage-dependent manner (9). Since sodium channel blockers (i.e., class I antiarrhythmic drugs) have been shown to increase the level of rat cardiac Na+ channel when used chronically, the inhibitory action of n − 3 PUFA on the Na+ currents raises the question of whether chronic supplementation with n − 3 PUFA would produce upregulation of cardiac Na+ channels similar to the findings of Duff and Catterall with the class I Na+ blocker, mexiletine (2, 3). Whether or not this effect occurs may determine the long-term outcome (efficacy and safety) of the antiarrhythmic therapy with the PUFA. At present, feeding studies in rats (10–12) and the clinical trials (13–15), unlike the Cardiac Arrhythmia Suppression Trial (1), have indicated an antiarrhythmic not arrhythmogenic effect in animals and humans chronically supplemented with n − 3 PUFA. Thus, we hypothesize that chronic supplementation with n − 3 PUFA may not increase or perhaps may suppress overexpression of cardiac Na+ channel. This study was intended to test this hypothesis.
We have used cultured neonatal rat cardiac myocytes to examine the effect of chronic supplementation of the cells with eicosapentaenoic acid (EPA, an n − 3 PUFA), mexiletine (a class I drug), or combination of EPA and mexiletine on the level of Na+ channel α-subunit mRNA by Northern blot hybridization and the density of Na+ channels by radioligand binding assay. We found that chronic EPA treatment not only fails to upregulate the cardiac sodium channel expression but also reduces the mexiletine-induced increase in the cardiac sodium channel expression.
MATERIALS AND METHODS
Cell Isolation and Culture.
Cardiac myocytes were isolated from 1-day-old neonatal Sprague–Dawley rats using the Neonatal Cardiomyocyte Isolation System (Worthington). The aortic root and atria were physically removed before isolation. The isolated cells were placed on Petri dishes with culture medium (F-10 nutrient mixture/10% horse serum/5% fetal bovine serum/50 μg/ml streptomycin/50 units/ml penicillin G) and cultured at 37°C in air with 5% CO2 added and 98% relative humidity in a tissue culture incubator (model 3123, Forma Scientific, Marietta, OH). The culture medium was changed every other day. After 48 hr in culture, cells exhibited regular spontaneous contractions. Cells were used for experiments after 3–6 days of culture.
Drug Treatment Protocols.
After 3 days in culture, when the myocytes normally form multicellular colonies or a monolayer of spontaneously beating cells, the culture medium was removed and replaced in the individual Petri dishes with fresh medium supplemented with 20 μM EPA, 20 μM mexiletine, 20 μM EPA plus 20 μM mexiletine, or placebo (ethanol). These concentrations were chosen based on their effective antiarrhythmic concentrations (5, 16). The cells were maintained in culture in this medium for an additional 4 days (3). Thereafter, the cultured medium was removed and the cells were washed and then used for determination of mRNA levels or for binding assay.
Separate experiments to test the effects of the monounsaturated and saturated fatty acids oleic acid and stearic acid (20 μM), respectively, were similarly done.
Batrachotoxinin [3H]Benzoate ([3H]BTXB) Radioligand Binding Assay.
Cells were detached from culture plates with 0.05% trypsin/EGTA (for 3 min at 37°C) and washed twice with the culture medium (as above). Cell viability was more than 95%, as measured by trypan blue exclusion. Cells were quantified by use of a cell counting chamber (hemocytometer).
Myocytes (5 × 105/assay) in 200 μl of incubation buffer (MEM with 50 μM CaCl2 and 1 mg/ml BSA) were incubated in duplicate or triplicate in polypropylene microcentrifuge tubes with 1.5 μM sea anemone toxin, 0.13 mM tetrodotoxin, and various concentrations of [3H]BTXB for 50–60 min at 37°C in the tissue-culture incubator. In standard protocol 30 nM [3H]BTXB was used. Tetrodotoxin was added to prevent depolarization induced by sodium influx (2, 17). Assays were done in parallel with tubes containing 0.4 mM aconitine to define nonspecific binding. At the end of the 60-min incubation period, 150 μl of the reaction mixture (cell suspension) from each sample was taken to filter through a Whatman GF/C 25-mm fiberglass filter under vacuum. Filters containing trapped myocytes were then quickly washed three times with 5 ml of ice-cold wash medium (140 mM NaCl/5 mM KCl/1.0 mM MgCl2/1.2 mM CaCl2/1.0 mM Na2HPO4/5.0 mM Hepes/10 mM glucose/1.0 mg/ml BSA, pH 7.4). The filters were then transferred to scintillation vials containing 10 ml of Ecoscint scintillation fluid (National Diagnostics), shaken by a Wrist Action shaker for 15 min, and assessed for cell-bound radioactivity in a Beckman LS-6000SC liquid-scintillation counter. (The counting efficiency was 48–50%.) Specific binding was calculated as the difference between total and nonspecific binding. Under these conditions nonspecific binding comprised 20–30% of total binding at 30 nM [3H]BTXB. The dissociation constant (Kd) and maximal binding (Bmax) were determined by Scatchard analysis (18).
Northern Blot Analysis.
Rat cardiac Na+ channel mRNA was determined by Northern analysis similar to that described by Duff and Catterall (3). Total cellular RNA was isolated using RNeasy Total RNA Kits (Qiagen, Chatsworth, CA). The concentration of the RNA is determined by measuring the A260 of an aliquot of the final preparation. RNA (10–20 μg for each sample) was electrophoresed in 1% agarose gels containing 2.2 M formaldehyde. The RNA in the gel was transferred by capillary elution to nitrocellulose filters, which were baked in vacuum to fix the RNA to the filters. Completeness of the transfer was verified by inspection of the ethidium bromide-stained gels. A labeled cRNA probe was made from a linearized plasmid Na-8.4 (generously provided by W. A. Catterall, University of Washington) containing an insert spanning nucleotides 3361–5868 of the α-subunit of the RIIA rat brain sodium channel (3), using Riboprobe in vitro Transcription System (Promega) with T7 RNA polymerase and [α-32P]UTP. Prehybridization, hybridization, and autoradiography were performed according to standard procedures (19). Radioactive signals (the autoradiographic band at 8.5 kb) were quantified using a laser densitometer.
To examine the specificity of the effect on sodium channel mRNA, nitrocellulose blots were subsequently probed with cDNA probes labeled with [α-32P]dCTP using a random primer technique from the cDNA clone encoding the constitutively expressed β-actin (a 700-bp fragment of the full-length cDNA). Thus, data are expressed as the ratio of densitometric integrals of the 8.5-kb band for the α-subunit of the sodium channel to that of β-actin.
Materials.
Neonatal Cardiomyocyte Isolation System Kit was purchased from Worthington. RNeasy Total RNA Kits was obtained from Qiagen. Riboprobe in vitro Transcription System was from Promega. [3H]BTXB (batrachotoxinin A 20-α-[2,5-3H]benzoate; 34.0 Ci/mmol, 1 Ci = 37 GBq), [α-32P]UTP, and [α-32P]dCTP were from New England Nuclear. Fatty acids, aconitine, and sea anemone toxins were obtained from Sigma. Tetrodotoxin was from Biomol (Plymouth Meeting, PA). Mexiletine was from Boehringer Ingelheim. Fatty acids were dissolved weekly in ethanol at the concentration of 10 mM and stored under a nitrogen atmosphere at −20°C. The Na-8.4 clone was a gift from W. A. Catterall (University of Washington).
RESULTS
Neonatal rat cardiac cells in culture for 2–3 days were then treated with the n − 3 PUFA EPA (20 μM), the class I antiarrhythmic drug mexiletine (20 μM), the combination of EPA (20 μM) and mexiletine (20 μM), or placebo (same amount of ethanol) for 3–4 days. The effects of these treatments on cardiac Na+ channel expression were assessed by comparison of both of the number of the Na+ channels per cell and the level of mRNA encoding Na+ channel α-subunit in the cells.
Effects of EPA and Mexiletine on the Number of Na+ Channels.
The number of sodium channels in rat cardiomyocytes was quantified by measuring the specific binding of the sodium channel-specific toxin [3H]BTXB to the cells. This has been shown to be a reliable method by a number of investigators (2, 16, 20, 21).
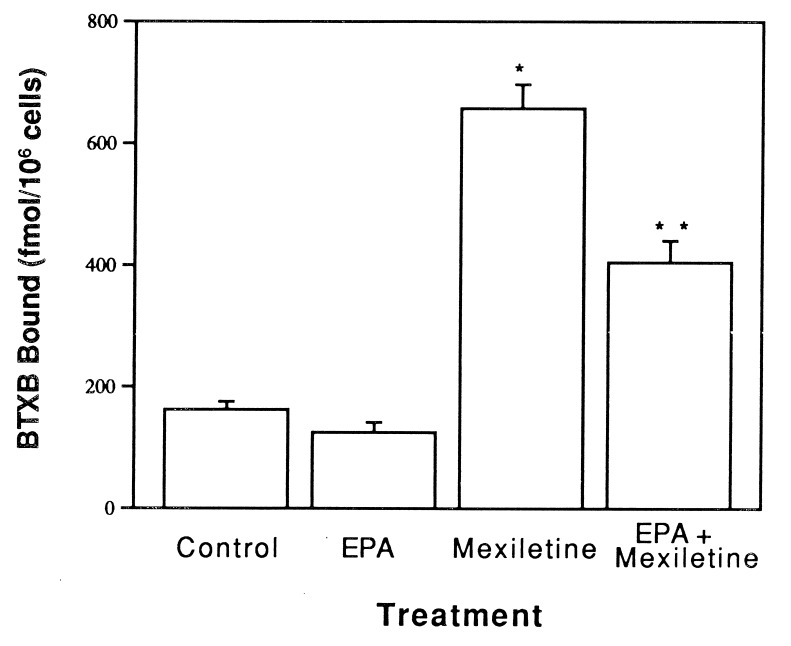
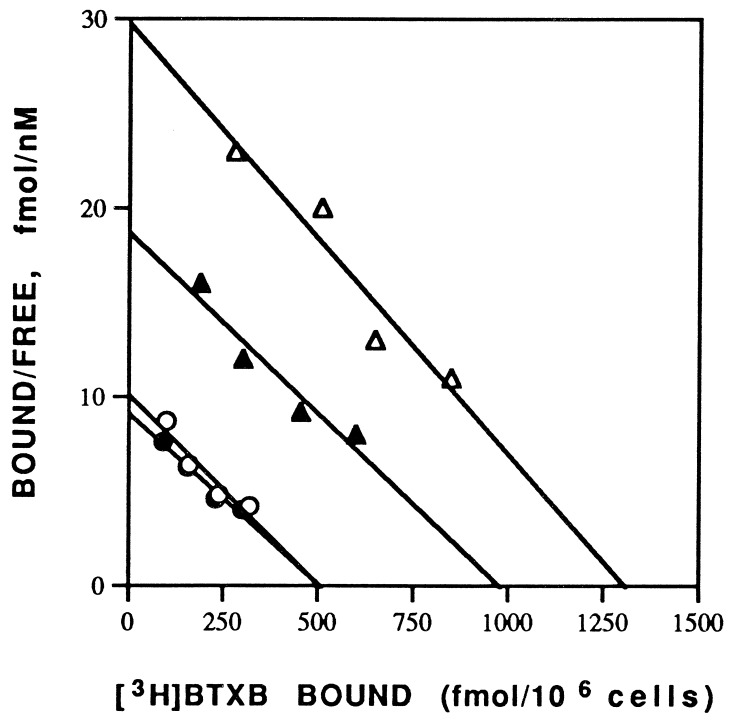
Fig. 1 shows the specific binding of [3H]BTXB (30 nM) to the same number of cells treated with different agents. Treatment of myocytes with mexiletine (20 μM) for 4 days induced a 4-fold increase in [3H]BTXB specific binding to the cells (658 ± 39 fmol/106 cells, n = 4) when compared with the control cells treated with placebo (162 ± 13 fmol/106 cells, n = 4). These results are consistent with the previous finding that the class I drug upregulates sodium channels (2, 3). Unlike the class I antiarrhythmic drug, EPA alone did not produce any significant effect on the binding of [3H]BTXB to the cells (125 ± 16 fmol/106 cells, n = 4). Interestingly, the combination of mexiletine and EPA resulted in a marked (≈40%) reduction in the [3H]BTXB binding (405 ± 36 fmol/106 cells, n = 4), compared with that seen with treatment with mexiletine alone (Fig. 1), indicating that the n − 3 PUFA (EPA) suppresses the mexiletine-induced increase in the cardiac sodium channel expression. Scatchard analysis indicates that the alterations of the [3H]BTXB binding by different treatments are due to an change in the maximal binding site without a change in the binding affinity (Fig. 2).
Figure 1.
Effect of supplementation with mexiletine, EPA, or EPA plus mexiletine on the number of sodium channels on neonatal rat myocytes. Cells were cultured in medium containing EPA (20 μM), mexiletine (20 μM), or both EPA (20 μM) and mexiletine (20 μM) for 4 days. The myocytes were then harvested, washed, and used for radioligand binding assay with 30 nM [3H]BTXB as described. The data are mean ± SE for four separate experiments (∗, P < 0.01 vs. control value; ∗∗, P < 0.05 vs. mexiletine value). Mexiletine increased the number of sodium channels per myocyte as indicated by the amount of [3H]BTXB binding, while EPA alone did not produce any significant effect on the binding of [3H]BTXB but significantly inhibited the upregulation effect of mexiletine.
Figure 2.
Scatchard analysis of [3H]BTXB binding to neonatal rat cardiomyocytes grown in the presence of different agents (with the same treatments as those described in Fig. 1). Binding of various concentrations of [3H]BTXB was measured as described in text. Each point represents the mean of duplicate determinations. Linear regression best-fit values for control (○), EPA (•), mexiletine (▵), and EPA plus mexiletine (▴) are Bmax = 506, 504, 1293, and 980 fmol, respectively, and Kd = 50, 55, 46, and 53 nM, respectively.
To test whether the monounsaturated fatty acid, oleic acid, and the saturated fatty acid, stearic acid, both of which lack an antiarrhythmic effect, have a similar effect on the mexiletine-induced upregulation of sodium channel expression, the cultured myocytes were treated with these fatty acids (20 μM) with or without mexiletine. The results indicate that these fatty acids, unlike EPA, did not exhibit a suppressing effect on mexiletine-induced upregulation of sodium channel expression. The binding of [3H]BTXB (20 nM) to the cells treated with placebo, oleic acid, or stearic acid was 110 ± 10, 114 ± 8, and 125 ± 12 (fmol/106 cells, n = 3), respectively, and the amounts of [3H]BTXB binding to the cells treated with mexiletine alone, mexiletine plus oleic acid, or mexiletine plus stearic acid were 389 ± 49, 391 ± 26, and 370 ± 27 (fmol/106 cells, n = 3), respectively.
Effects of EPA and Mexiletine on the Level of mRNA Encoding the α-Subunit of the Sodium Channel.
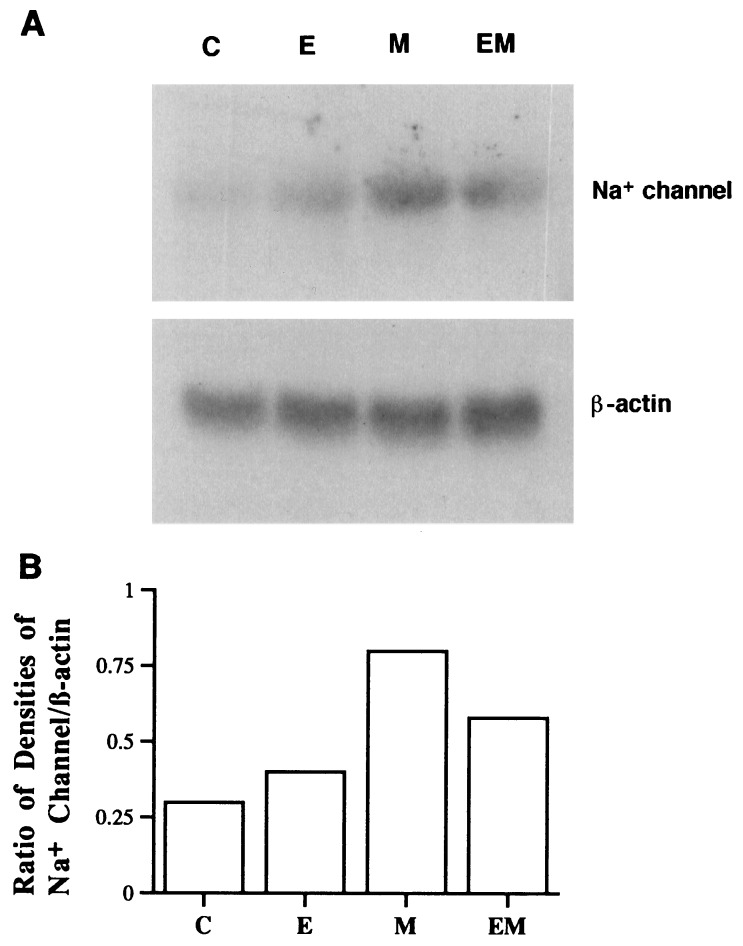
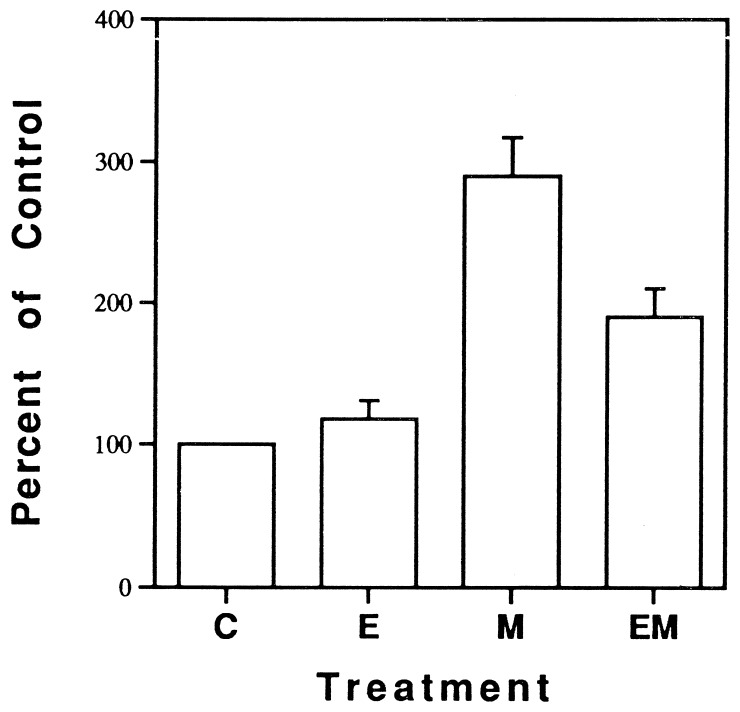
RNA isolated from cardiac myocytes treated with various agents was probed with a 2.5-kb cRNA transcribed with T7 RNA polymerase from the clone Na-8.4, which encodes nucleotides 3361–5868 of the α-subunit of the RIIA sodium channel subtype from rat brain. Previous studies have reported substantial homology among sodium channels in rat skeletal muscle, brain and heart in these homologous domains (3, 22). Fig. 3 shows a representative Northern blot hybridization of mRNA encoding the cardiac sodium channel, from cultured myocytes treated with placebo, EPA, mexiletine, or mexiletine plus EPA. Mexiletine produced a significant increase in mRNA level encoding the sodium channel, whereas EPA alone had no significant effect, but, when combined with mexiletine, EPA suppressed the increase in the mRNA level induced by mexiletine (Fig. 3). In contrast, the level of mRNA encoding β-actin on the same blot was almost unchanged by the treatments (Fig. 3), indicating that the effect on sodium channel mRNA is specific and is not due to overall changes in mRNA level or variability in gel loading. Data from three experiments expressed as percentage of control based on the ratio of densitometric integrals of the 8.5-kb band for the α-subunit of the sodium channel to that of β-actin is shown in Fig. 4. The changes in the level of mRNA encoding sodium channel α-subunit were well correlated with comparable changes in sodium channel number (the [3H]BTXB binding shown in Fig. 1) in the cultured myocytes.
Figure 3.
(A) Northern blot hybridization of mRNA encoding cardiac sodium channel (Upper) and mRNA encoding β-actin (Lower, as references) in rat cardiac myocytes treated with placebo (C), 20 μM EPA (E), 20 μM mexiletine (M), or EPA plus mexiletine (EM) for 4 days. (B) Ratio of the densitometric integral of the α-subunit sodium channel band to that of β-actin. Treatment with mexiletine increased the amount of sodium channel mRNA while EPA alone did not significantly change the mRNA level but suppressed the effect of mexiletine.
Figure 4.
Mean data of mRNA levels from all results of Northern blot analysis (n = 3) are expressed as a percentage of control, based on ratio of the densitometric integral of the α-subunit sodium channel band to that of β-actin. C, control; E, EPA; M, mexiletine; EM, EPA plus mexiletine.
DISCUSSION
The present study has shown that supplementation of cultured cardiac myocytes with mexiletine, a class I antiarrhythmic drug that blocks cardiac sodium channels, increased both the number of sodium channels and the level of mRNA encoding the channel α-subunit in the cells whereas supplementation of the myocytes with EPA, an n − 3 polyunsaturated fatty acid that also blocks the sodium channels, failed to do so. Thus, these findings support our hypotheses that n − 3 PUFA when used chronically do not mimic the effect of class I antiarrhythmic drugs to up-regulate cardiac Na+ channel expression despite their similar blocking effect on the sodium channels.
The results of the present study suggest that cardiac sodium channels are under dynamic change and that certain drugs or nutrients can modulate their expression. The similarity between the changes in mRNA levels and sodium channel density indicates that regulation of transcription of mRNA or its processing and stability is primarily responsible for the regulation of sodium channel number.
Sodium channel blockers have been previously shown to up-regulate sodium channel expression, as measured by Northern blot analysis and radioligand binding assay (2, 3, 22, 23). Chronic in vivo treatment with mexiletine produced an increase in both sodium channel mRNA and protein in rat cardiac muscle (2, 3). Similar results were found in skeletal muscle cells in culture treated with bupivacaine, another sodium channel blocker (22, 23). The effects of mexiletine in cultured cardiac myocytes observed in the present study are consistent with these previous findings. One mechanism proposed by these authors is that alterations in intracellular calcium level regulate sodium channel expression. Whether the PUFA reduce cytosolic free calcium or not remains to be determined. If they do reduce cytosolic calcium levels, then clearly other effects of the PUFA must prevent the overexpression of Na+ channels. Unlike mexiletine that affects only sodium channels, n − 3 PUFA have been shown to modulate a number of other ion channels (for review, see ref. 4) and regulate many cellular processes that are related to gene expression such as signal transduction, DNA-protein interaction, cellular acidification, etc. (24). The multiple (diverse) biological effects of n − 3 fatty acids render them able to affect gene expression through different pathways or at different levels. In fact, PUFA have recently been shown to suppress the expression of a number of genes in other tissues (25–29), although the mechanism of their action remains unclear.
The finding that these fatty acids can reduce the overexpression of sodium channels induced by the class I drugs suggests a novel beneficial effect of n − 3 PUFA. They may also inhibit up-regulation of cardiac sodium channel numbers from other causes. There are other potential factors that may modulate ion channel gene expression such as repetitive activity, depolarization, protein kinase activation, or elevation of intracellular calcium (22, 30). Such modifications may create a clinical arrhythmogenic substrate in diseases such as coronary heart disease or chronic congestive heart failure. Thus, if an increase in cardiac sodium channel number is indeed an arrhythmogenic substrate as suggested by Duff and coworkers (2, 3), the inhibitory effect of n − 3 PUFA on such modulatory mechanisms to prevent up-regulation of sodium channels would add additional value to these fatty acids as antiarrhythmic agents. That is, n − 3 PUFA may achieve effective antiarrhythmic effects through both an acute effect on ion channel conductance and a long-term effect on gene expression.
Malignant cardiac arrhythmias such as ventricular tachycardia and ventricular fibrillation are the underlying cause of sudden cardiac death, which accounts for 50–60% (some 250,000 annually in the U.S. alone) of deaths from acute myocardial infarction (31). Nevertheless, the existing common pharmacological interventions such as the ion channel blockers, when administrated chronically, have been proven unsafe and ineffective (30). Thus, finding a safe and highly effective means of preventing fatal arrhythmias may have significant public health benefits. Although n − 3 PUFA have recently been shown with growing evidence from in vitro as well as in vivo studies to have an effective antiarrhythmic action, without full assessment of their action including acute and long-term effects, the clinical utility of these fatty acids as pharmaceutical agents for cardiac arrhythmias will be hampered. Since the nature of n − 3 PUFA’s effects on cardiac Na+ channel expression may determine the long-term outcome (efficacy and safety) of the antiarrhythmic therapy with the PUFA, the present study showing that chronic PUFA treatment not only does not up-regulate the cardiac sodium channel expression but also suppresses the increase in the cardiac sodium channel expression induced by other factors should add important insight into the benefits and advantage of clinical application of n − 3 fatty acids as novel antiarrhythmic agents.
Acknowledgments
We thank Dr. W. A. Catterall for providing the Na-8.4 clone. We are grateful to Amy Howard for her technical assistance. This study was supported by National Institutes of Health Grants from the National Institute of Diabetes and Digestive and Kidney Diseases (RO1-DK38165) to A.L. and from National Institutes of Health–Clinical Nutrition Research Center at Harvard University (P30-DK40561) to J.X.K.
ABBREVIATIONS
- BTXB
batrachotoxinin benzoate
- EPA
eicosapentaenoic acid
- PUFA
polyunsaturated fatty acids
References
- 1.The Cardiac Arrhythmia Suppression Trial Investigators. N Engl J Med. 1989;321:406–412. doi: 10.1056/NEJM198912213212510. [DOI] [PubMed] [Google Scholar]
- 2.Taouis M, Sheldon R S, Duff H J. J Clin Invest. 1991;88:375–378. doi: 10.1172/JCI115313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Duff H J, Offord J, West J, Catterall W A. Mol Pharmacol. 1992;42:570–574. [PubMed] [Google Scholar]
- 4.Kang J X, Leaf A. Circulation. 1996;94:1774–1780. doi: 10.1161/01.cir.94.7.1774. [DOI] [PubMed] [Google Scholar]
- 5.Kang J X, Leaf A. Proc Natl Acad Sci USA. 1994;91:9886–9890. doi: 10.1073/pnas.91.21.9886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kang J X, Leaf A. Biochem Biophys Res Commun. 1995;208:629–636. doi: 10.1006/bbrc.1995.1385. [DOI] [PubMed] [Google Scholar]
- 7.Kang J X, Leaf A. Eur J Pharmacol. 1996;297:97–106. doi: 10.1016/0014-2999(95)00701-6. [DOI] [PubMed] [Google Scholar]
- 8.Kang J X, Xiao Y F, Leaf A. Proc Natl Acad Sci USA. 1995;92:3997–4001. doi: 10.1073/pnas.92.9.3997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xiao Y F, Kang J X, Morgan J P, Leaf A. Proc Natl Acad Sci USA. 1995;92:11000–11004. doi: 10.1073/pnas.92.24.11000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McLennan P L, Abeywardena M Y, Charnock J S. Can J Physiol Pharmacol. 1985;63:1411–1417. doi: 10.1139/y85-232. [DOI] [PubMed] [Google Scholar]
- 11.McLennan P L, Abeywardena M Y, Charnock J S. Am Heart J. 1988;16:709–717. doi: 10.1016/0002-8703(88)90328-6. [DOI] [PubMed] [Google Scholar]
- 12.McLennan P L, Bridle T M, Abeywardena M Y, Charnock J S. Am Heart J. 1992;123:1555–1561. doi: 10.1016/0002-8703(92)90809-a. [DOI] [PubMed] [Google Scholar]
- 13.de Logeril M, Renaud S, Mamelle N, Salen P, Martin J-L, Monjaud I, Guidollet J, Touboul P, Delaye J. Lancet. 1994;343:1454–1459. doi: 10.1016/s0140-6736(94)92580-1. [DOI] [PubMed] [Google Scholar]
- 14.Burr M L, Gilbert J F, Holliday R M, Elwood P C, Fehily A M, Rogers S, Sweetnam P M, Deadman N M. Lancet. 1989;334:757–761. doi: 10.1016/s0140-6736(89)90828-3. [DOI] [PubMed] [Google Scholar]
- 15.Siscovick D S, Raghunathan T E, King I, Weinmann S, Wicklund K G, Albright J, Bovbjerg V, Arbogast P, Smith H, Kushi L. J Am Med Assoc. 1995;274:1363–1367. doi: 10.1001/jama.1995.03530170043030. [DOI] [PubMed] [Google Scholar]
- 16.Sheldon R S, Cannon N J, Duff H J. Circ Res. 1987;61:492–497. doi: 10.1161/01.res.61.4.492. [DOI] [PubMed] [Google Scholar]
- 17.Catterall W A. Annu Rev Pharmacol Toxicol. 1980;20:15–43. doi: 10.1146/annurev.pa.20.040180.000311. [DOI] [PubMed] [Google Scholar]
- 18.Scatchard G. Ann NY Acad Sci. 1949;51:660–672. [Google Scholar]
- 19.Sambrook J, Fritsch E F, Maniatis T. Molecular Cloning: A Laboratory Manual. 2nd Ed. Plainview, NY: Cold Spring Harbor Lab. Press; 1989. [Google Scholar]
- 20.Postma S W, Catterall W A. Mol Pharmacol. 1984;25:219–227. [PubMed] [Google Scholar]
- 21.Sheldon R S, Cannon N J, Duff H J. Mol Pharmacol. 1986;30:617–623. [PubMed] [Google Scholar]
- 22.Offord J, Catterall W A. Neuron. 1989;2:1447–1452. doi: 10.1016/0896-6273(89)90190-6. [DOI] [PubMed] [Google Scholar]
- 23.Sherman S J, Catterall W A. Proc Natl Acad Sci USA. 1984;81:262–266. doi: 10.1073/pnas.81.1.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baillie R A, Jump D B, Clarke S D. Curr Opin Lipidol. 1996;7:53–55. doi: 10.1097/00041433-199602000-00012. [DOI] [PubMed] [Google Scholar]
- 25.Clark S D, Jump D B. Annu Rev Nutr. 1994;14:83–98. doi: 10.1146/annurev.nu.14.070194.000503. [DOI] [PubMed] [Google Scholar]
- 26.Robinson D R, Urakaze M, Huang R, Taki H, Sugiyama E C, Knoell T, Xu L L, Yeh E T H, Auron P E. Lipids. 1996;31:S23–S32. doi: 10.1007/BF02637046. [DOI] [PubMed] [Google Scholar]
- 27.Clandinin M T, Cheema S, Pehowich D, Field C J. Lipids. 1996;31:S13–S22. doi: 10.1007/BF02637045. [DOI] [PubMed] [Google Scholar]
- 28.Waters K M, Natambi J M. Lipids. 1996;31:S33–S26. doi: 10.1007/BF02637047. [DOI] [PubMed] [Google Scholar]
- 29.Sellmayer A, Danesch U, Weber P C. Lipids. 1996;31:S37–S40. doi: 10.1007/BF02637048. [DOI] [PubMed] [Google Scholar]
- 30.Roden D M, Tamkun M M. Trends Cardiovasc Med. 1994;4:278–285. doi: 10.1016/1050-1738(94)90032-9. [DOI] [PubMed] [Google Scholar]
- 31.American Heart Association. Heart and Stroke Facts: Statistical Supplement. Dallas: Am. Heart Assn.; 1995. [Google Scholar]