Abstract
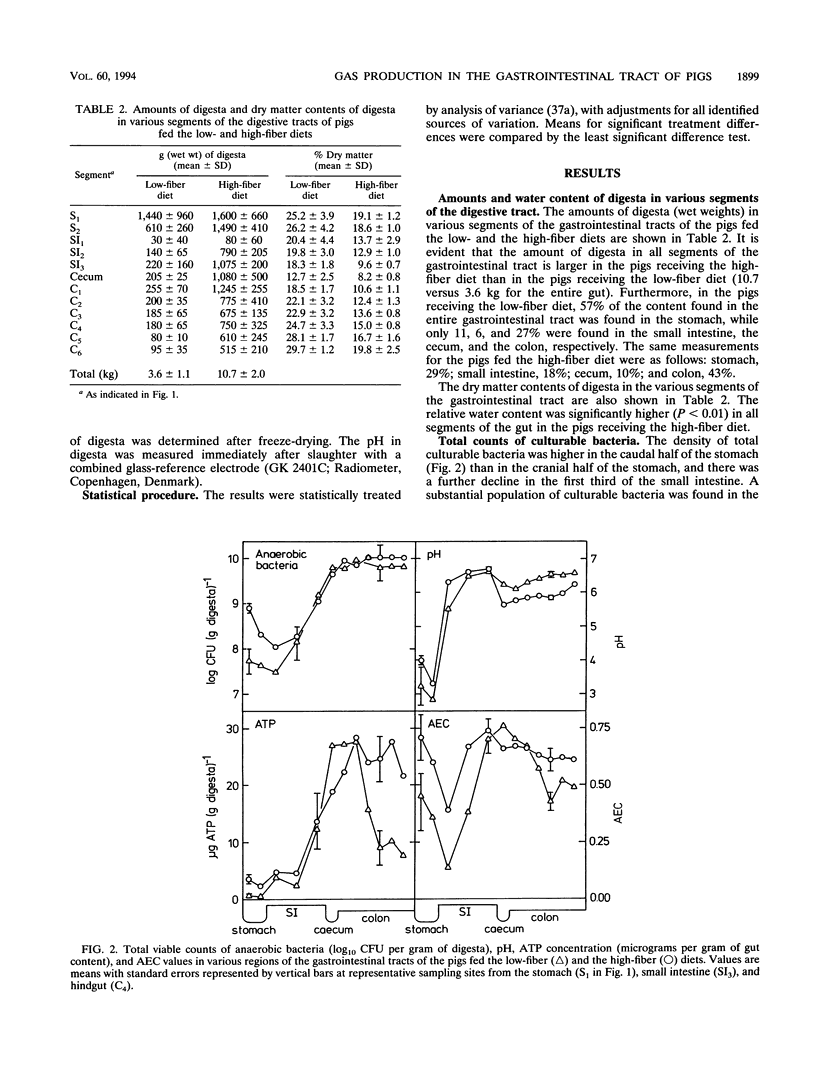
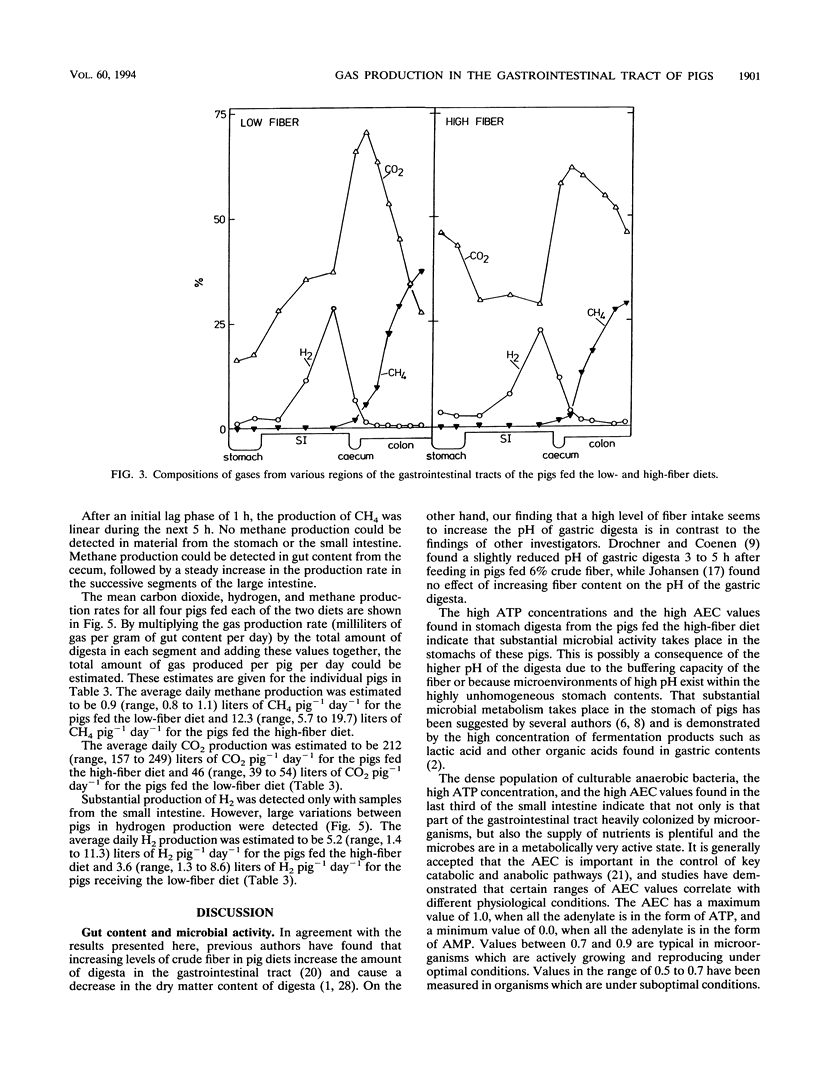
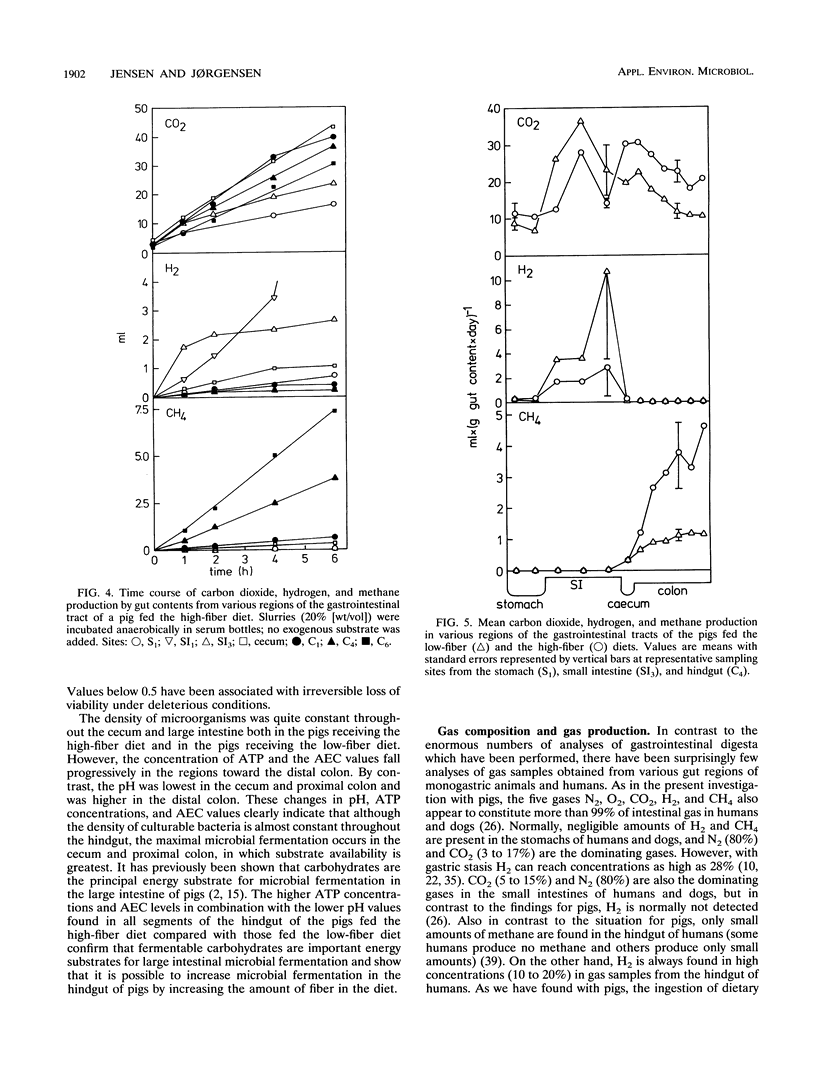
The microbial activity, composition of the gas phase, and gas production rates in the gastrointestinal tract of pigs fed either a low- or a high-fiber diet were investigated. Dense populations of culturable anaerobic bacteria, high ATP concentrations, and high adenylate energy charges were found for the last third of the small intestine, indicating that substantial microbial activity takes place in that portion of the gut. The highest microbial activity (highest bacterium counts, highest ATP concentration, high adenylate energy charge, and low pH) was found in the cecum and proximal colon. Greater microbial activity was found in the stomach and all segments of the hindgut in the pigs fed the high-fiber diet than in the pigs fed the low-fiber diet. Considerable amounts of O2 were found in the stomach (around 5%), while the content of O2 in gas samples taken from all other parts of the gastrointestinal tract was < 1%. The highest concentrations and highest production rates for H2 were found in the last third of the small intestine. No methane could be detected in the stomach or the small intestine. The rate of production and concentration of methane in the cecum and the proximal colon were low, followed by a steady increase in the successive segments of the hindgut. A very good correlation between in vivo and in vitro measurements of methane production was found. The amount of CH4 produced by pigs fed the low-fiber diet was 1.4 liters/day per animal. Substantially larger amounts of CH4 were produced by pigs fed the high-fiber diet (12.5 liters/day)(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bach Knudsen K. E., Hansen I. Gastrointestinal implications in pigs of wheat and oat fractions. 1. Digestibility and bulking properties of polysaccharides and other major constituents. Br J Nutr. 1991 Mar;65(2):217–232. doi: 10.1079/bjn19910082. [DOI] [PubMed] [Google Scholar]
- Bach Knudsen K. E., Jensen B. B., Andersen J. O., Hansen I. Gastrointestinal implications in pigs of wheat and oat fractions. 2. Microbial activity in the gastrointestinal tract. Br J Nutr. 1991 Mar;65(2):233–248. doi: 10.1079/bjn19910083. [DOI] [PubMed] [Google Scholar]
- Chapman A. G., Atkinson D. E. Adenine nucleotide concentrations and turnover rates. Their correlation with biological activity in bacteria and yeast. Adv Microb Physiol. 1977;15:253–306. doi: 10.1016/s0065-2911(08)60318-5. [DOI] [PubMed] [Google Scholar]
- Christensen K., Thorbek G. Methane excretion in the growing pig. Br J Nutr. 1987 May;57(3):355–361. doi: 10.1079/bjn19870043. [DOI] [PubMed] [Google Scholar]
- Cranwell P. D., Noakes D. E., Hill K. J. Gastric secretion and fermentation in the suckling pig. Br J Nutr. 1976 Jul;36(1):71–86. doi: 10.1079/bjn19760059. [DOI] [PubMed] [Google Scholar]
- De Graeve K. G., Grivet J. P., Durand M., Beaumatin P., Demeyer D. NMR study of 13CO2 incorporation into short-chain fatty acids by pig large-intestinal flora. Can J Microbiol. 1990 Aug;36(8):579–582. doi: 10.1139/m90-101. [DOI] [PubMed] [Google Scholar]
- Gibson G. R., Macfarlane G. T., Cummings J. H. Occurrence of sulphate-reducing bacteria in human faeces and the relationship of dissimilatory sulphate reduction to methanogenesis in the large gut. J Appl Bacteriol. 1988 Aug;65(2):103–111. doi: 10.1111/j.1365-2672.1988.tb01498.x. [DOI] [PubMed] [Google Scholar]
- Karl D. M. Cellular nucleotide measurements and applications in microbial ecology. Microbiol Rev. 1980 Dec;44(4):739–796. doi: 10.1128/mr.44.4.739-796.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khin-Maung-U, Tin-Aye, Ku-Tin-Myint, Tin-Oo, Myo-Khin, Thackway S. A., Connor S. J., Bolin T. D., Duncombe V. M. In vitro hydrogen production by enteric bacteria cultured from children with small bowel bacterial overgrowth. J Pediatr Gastroenterol Nutr. 1992 Feb;14(2):192–197. doi: 10.1097/00005176-199202000-00013. [DOI] [PubMed] [Google Scholar]
- Lajoie S. F., Bank S., Miller T. L., Wolin M. J. Acetate production from hydrogen and [13C]carbon dioxide by the microflora of human feces. Appl Environ Microbiol. 1988 Nov;54(11):2723–2727. doi: 10.1128/aem.54.11.2723-2727.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitt M. D., Donaldson R. M. Use of respiratory hydrogen (H2) excretion to detect carbohydrate malabsorption. J Lab Clin Med. 1970 Jun;75(6):937–945. [PubMed] [Google Scholar]
- Low A. G., Partridge I. G., Sambrook I. E. Studies on digestion and absorption in the intestines of growing pigs. 2. Measurements of the flow of dry matter, ash and water. Br J Nutr. 1978 May;39(3):515–526. doi: 10.1079/bjn19780067. [DOI] [PubMed] [Google Scholar]
- McElroy W. D. The Energy Source for Bioluminescence in an Isolated System. Proc Natl Acad Sci U S A. 1947 Nov;33(11):342–345. doi: 10.1073/pnas.33.11.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller T. L., Wolin M. J. A serum bottle modification of the Hungate technique for cultivating obligate anaerobes. Appl Microbiol. 1974 May;27(5):985–987. doi: 10.1128/am.27.5.985-987.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller T. L., Wolin M. J. Fermentations by saccharolytic intestinal bacteria. Am J Clin Nutr. 1979 Jan;32(1):164–172. doi: 10.1093/ajcn/32.1.164. [DOI] [PubMed] [Google Scholar]
- Rhodes J. M., Middleton P., Jewell D. P. The lactulose hydrogen breath test as a diagnostic test for small-bowel bacterial overgrowth. Scand J Gastroenterol. 1979;14(3):333–336. doi: 10.3109/00365527909179892. [DOI] [PubMed] [Google Scholar]
- Robinson J. A., Smolenski W. J., Ogilvie M. L., Peters J. P. In vitro total-gas, CH4, H2, volatile fatty acid, and lactate kinetics studies on luminal contents from the small intestine, cecum, and colon of the pig. Appl Environ Microbiol. 1989 Oct;55(10):2460–2467. doi: 10.1128/aem.55.10.2460-2467.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roediger W. E. Role of anaerobic bacteria in the metabolic welfare of the colonic mucosa in man. Gut. 1980 Sep;21(9):793–798. doi: 10.1136/gut.21.9.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver G. A., Krause J. A., Miller T. L., Wolin M. J. Constancy of glucose and starch fermentations by two different human faecal microbial communities. Gut. 1989 Jan;30(1):19–25. doi: 10.1136/gut.30.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolever T. M., Cohen Z., Thompson L. U., Thorne M. J., Jenkins M. J., Prokipchuk E. J., Jenkins D. J. Ileal loss of available carbohydrate in man: comparison of a breath hydrogen method with direct measurement using a human ileostomy model. Am J Gastroenterol. 1986 Feb;81(2):115–122. [PubMed] [Google Scholar]



