Abstract
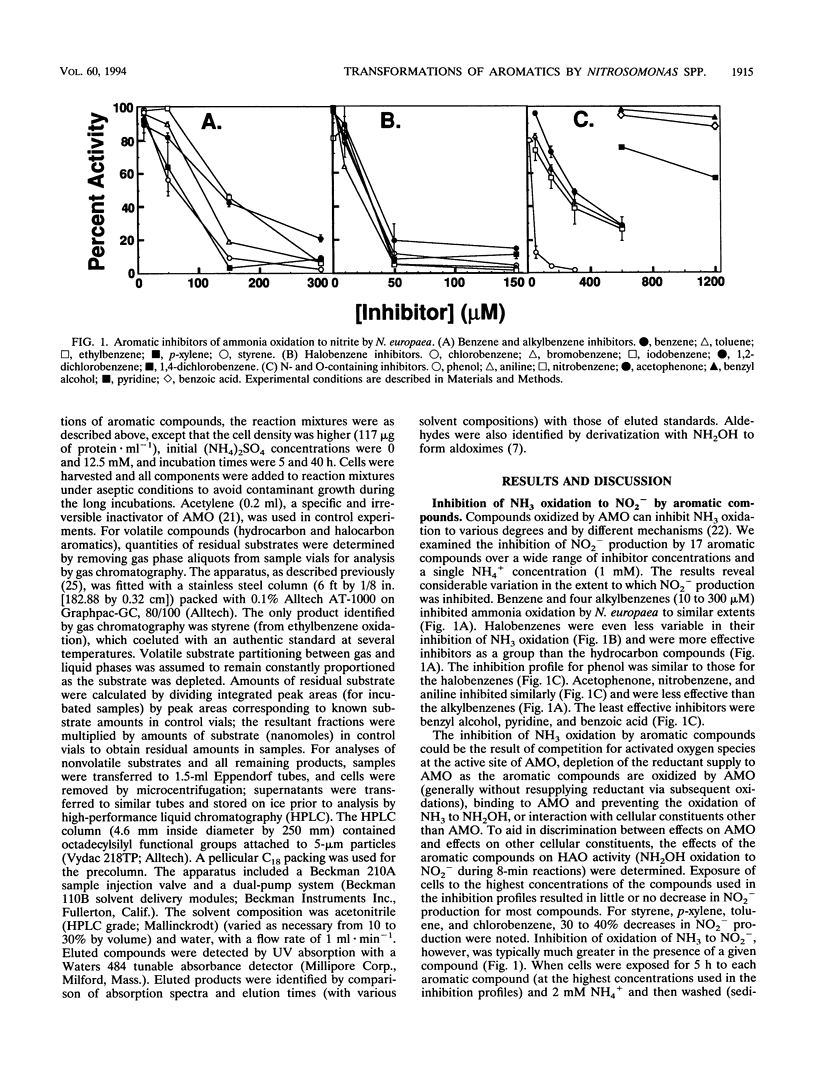
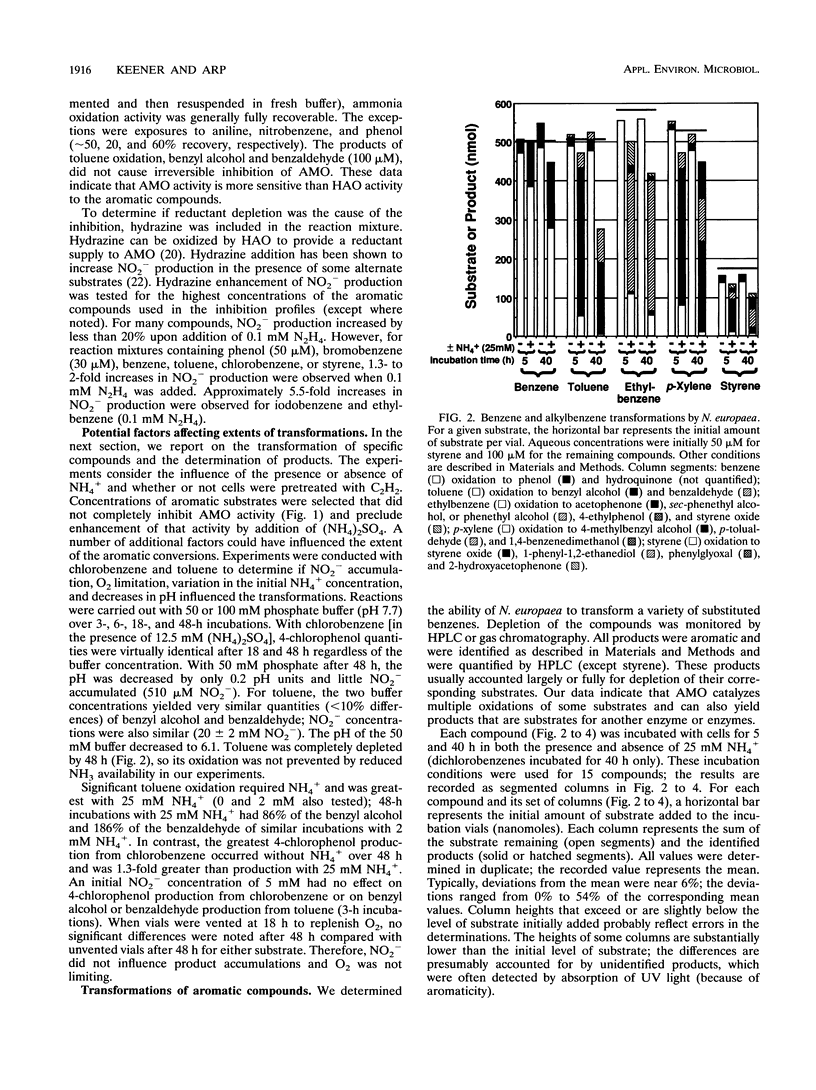
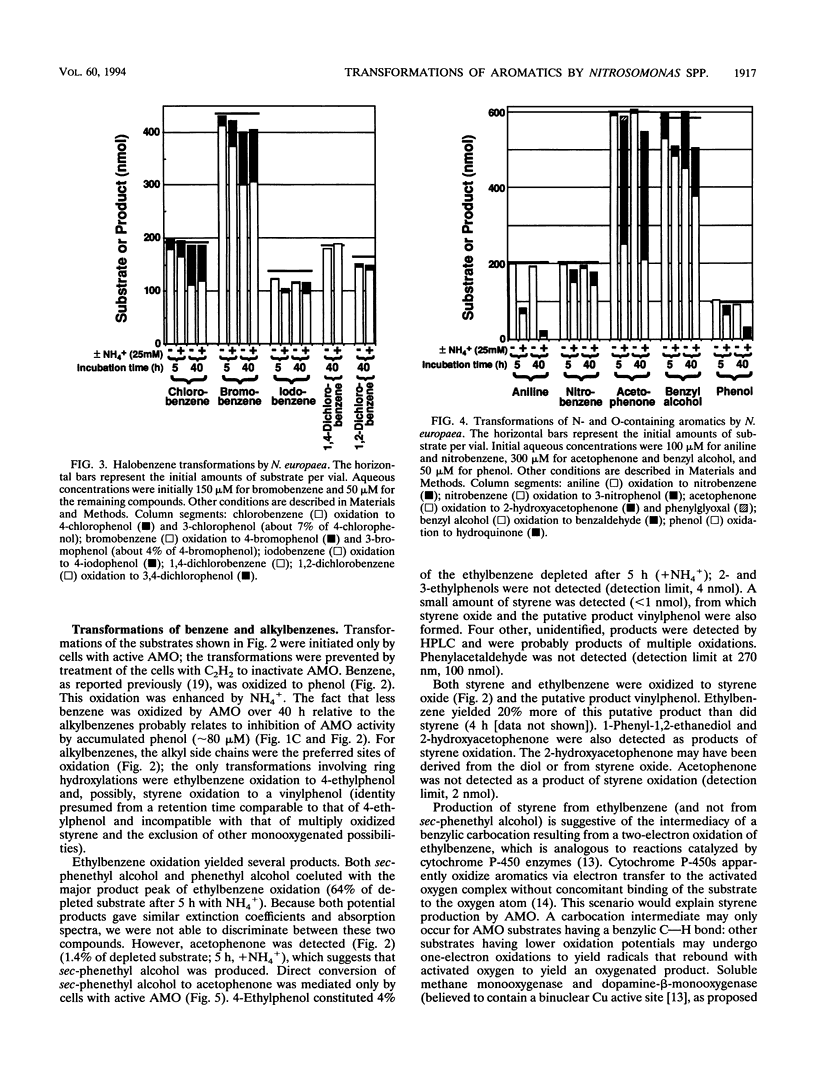
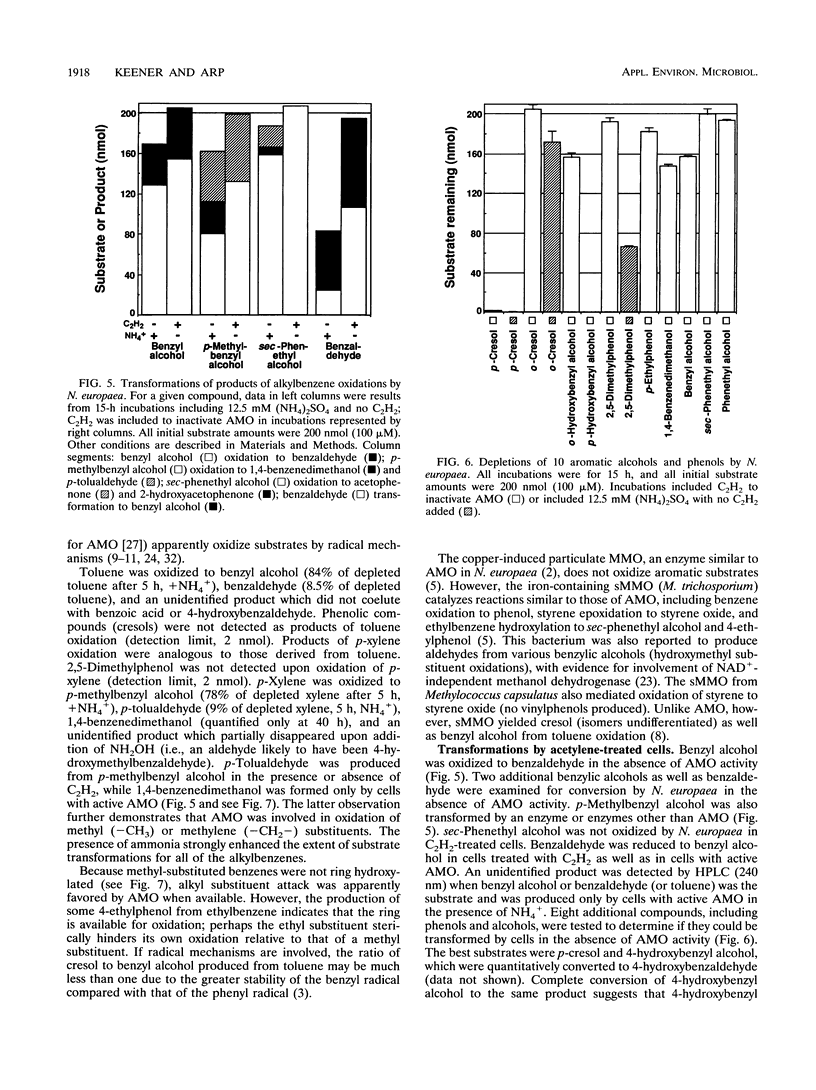
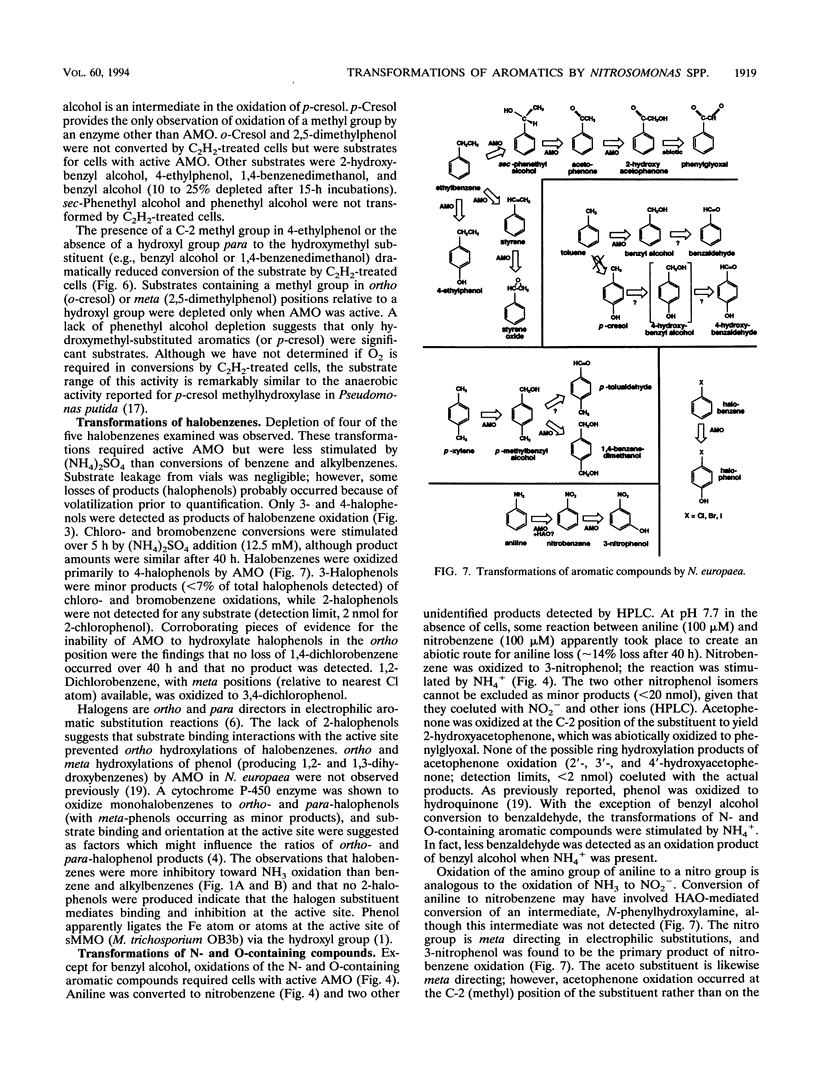
Benzene and a variety of substituted benzenes inhibited ammonia oxidation by intact cells of Nitrosomonas europaea. In most cases, the inhibition was accompanied by transformation of the aromatic compound to a more oxidized product or products. All products detected were aromatic, and substituents were often oxidized but were not separated from the benzene ring. Most transformations were enhanced by (NH4)2SO4 (12.5 mM) and were prevented by C2H2, a mechanism-based inactivator of ammonia monooxygenase (AMO). AMO catalyzed alkyl substituent hydroxylations, styrene epoxidation, ethylbenzene desaturation to styrene, and aniline oxidation to nitrobenzene (and unidentified products). Alkyl substituents were preferred oxidation sites, but the ring was also oxidized to produce phenolic compounds from benzene, ethylbenzene, halobenzenes, phenol, and nitrobenzene. No carboxylic acids were identified. Ethylbenzene was oxidized via styrene to two products common also to oxidation of styrene; production of styrene is suggestive of an electron transfer mechanism for AMO. Iodobenzene and 1,2-dichlorobenzene were oxidized slowly to halophenols; 1,4-dichlorobenzene was not transformed. No 2-halophenols were detected as products. Several hydroxymethyl (-CH2OH)-substituted aromatics and p-cresol were oxidized by C2H2-treated cells to the corresponding aldehydes, benzaldehyde was reduced to benzyl alcohol, and o-cresol and 2,5-dimethylphenol were not depleted.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burka L. T., Plucinski T. M., Macdonald T. L. Mechanisms of hydroxylation by cytochrome P-450: metabolism of monohalobenzenes by phenobarbital-induced microsomes. Proc Natl Acad Sci U S A. 1983 Nov;80(21):6680–6684. doi: 10.1073/pnas.80.21.6680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bédard C., Knowles R. Physiology, biochemistry, and specific inhibitors of CH4, NH4+, and CO oxidation by methanotrophs and nitrifiers. Microbiol Rev. 1989 Mar;53(1):68–84. doi: 10.1128/mr.53.1.68-84.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colby J., Stirling D. I., Dalton H. The soluble methane mono-oxygenase of Methylococcus capsulatus (Bath). Its ability to oxygenate n-alkanes, n-alkenes, ethers, and alicyclic, aromatic and heterocyclic compounds. Biochem J. 1977 Aug 1;165(2):395–402. doi: 10.1042/bj1650395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzpatrick P. F., Villafranca J. J. Mechanism-based inhibitors of dopamine beta-hydroxylase. Arch Biochem Biophys. 1987 Sep;257(2):231–250. doi: 10.1016/0003-9861(87)90563-7. [DOI] [PubMed] [Google Scholar]
- Fox B. G., Borneman J. G., Wackett L. P., Lipscomb J. D. Haloalkene oxidation by the soluble methane monooxygenase from Methylosinus trichosporium OB3b: mechanistic and environmental implications. Biochemistry. 1990 Jul 10;29(27):6419–6427. doi: 10.1021/bi00479a013. [DOI] [PubMed] [Google Scholar]
- Fox B. G., Froland W. A., Dege J. E., Lipscomb J. D. Methane monooxygenase from Methylosinus trichosporium OB3b. Purification and properties of a three-component system with high specific activity from a type II methanotroph. J Biol Chem. 1989 Jun 15;264(17):10023–10033. [PubMed] [Google Scholar]
- Guengerich F. P. Enzymatic oxidation of xenobiotic chemicals. Crit Rev Biochem Mol Biol. 1990;25(2):97–153. doi: 10.3109/10409239009090607. [DOI] [PubMed] [Google Scholar]
- Guengerich F. P., MacDonald T. L. Mechanisms of cytochrome P-450 catalysis. FASEB J. 1990 May;4(8):2453–2459. doi: 10.1096/fasebj.4.8.2185971. [DOI] [PubMed] [Google Scholar]
- Hooper A. B., Terry K. R. Specific inhibitors of ammonia oxidation in Nitrosomonas. J Bacteriol. 1973 Aug;115(2):480–485. doi: 10.1128/jb.115.2.480-485.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman M. R., Murton I. B., Arp D. J. Interaction of Ammonia Monooxygenase from Nitrosomonas europaea with Alkanes, Alkenes, and Alkynes. Appl Environ Microbiol. 1988 Dec;54(12):3187–3190. doi: 10.1128/aem.54.12.3187-3190.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman M. R., Wood P. M. Methane oxidation by Nitrosomonas europaea. Biochem J. 1983 Apr 15;212(1):31–37. doi: 10.1042/bj2120031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman M. R., Wood P. M. Suicidal inactivation and labelling of ammonia mono-oxygenase by acetylene. Biochem J. 1985 May 1;227(3):719–725. doi: 10.1042/bj2270719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keener W. K., Arp D. J. Kinetic Studies of Ammonia Monooxygenase Inhibition in Nitrosomonas europaea by Hydrocarbons and Halogenated Hydrocarbons in an Optimized Whole-Cell Assay. Appl Environ Microbiol. 1993 Aug;59(8):2501–2510. doi: 10.1128/aem.59.8.2501-2510.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mountfort D. O., White D., Asher R. A. Oxidation of Lignin-Related Aromatic Alcohols by Cell Suspensions of Methylosinus trichosporium. Appl Environ Microbiol. 1990 Jan;56(1):245–249. doi: 10.1128/aem.56.1.245-249.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasche M. E., Hicks R. E., Hyman M. R., Arp D. J. Oxidation of monohalogenated ethanes and n-chlorinated alkanes by whole cells of Nitrosomonas europaea. J Bacteriol. 1990 Sep;172(9):5368–5373. doi: 10.1128/jb.172.9.5368-5373.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasche M. E., Hyman M. R., Arp D. J. Factors Limiting Aliphatic Chlorocarbon Degradation by Nitrosomonas europaea: Cometabolic Inactivation of Ammonia Monooxygenase and Substrate Specificity. Appl Environ Microbiol. 1991 Oct;57(10):2986–2994. doi: 10.1128/aem.57.10.2986-2994.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shears J. H., Wood P. M. Spectroscopic evidence for a photosensitive oxygenated state of ammonia mono-oxygenase. Biochem J. 1985 Mar 1;226(2):499–507. doi: 10.1042/bj2260499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stafford D. A. The effect of phenols and heterocyclic bases on nitrification in activated sludges. J Appl Bacteriol. 1974 Mar;37(1):75–82. doi: 10.1111/j.1365-2672.1974.tb00416.x. [DOI] [PubMed] [Google Scholar]
- Tomlinson T. G., Boon A. G., Trotman C. N. Inhibition of nitrification in the activated sludge process of sewage disposal. J Appl Bacteriol. 1966 Aug;29(2):266–291. doi: 10.1111/j.1365-2672.1966.tb03477.x. [DOI] [PubMed] [Google Scholar]
- Vannelli T., Hooper A. B. Oxidation of Nitrapyrin to 6-Chloropicolinic Acid by the Ammonia-Oxidizing Bacterium Nitrosomonas europaea. Appl Environ Microbiol. 1992 Jul;58(7):2321–2325. doi: 10.1128/aem.58.7.2321-2325.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vannelli T., Logan M., Arciero D. M., Hooper A. B. Degradation of halogenated aliphatic compounds by the ammonia- oxidizing bacterium Nitrosomonas europaea. Appl Environ Microbiol. 1990 Apr;56(4):1169–1171. doi: 10.1128/aem.56.4.1169-1171.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]



