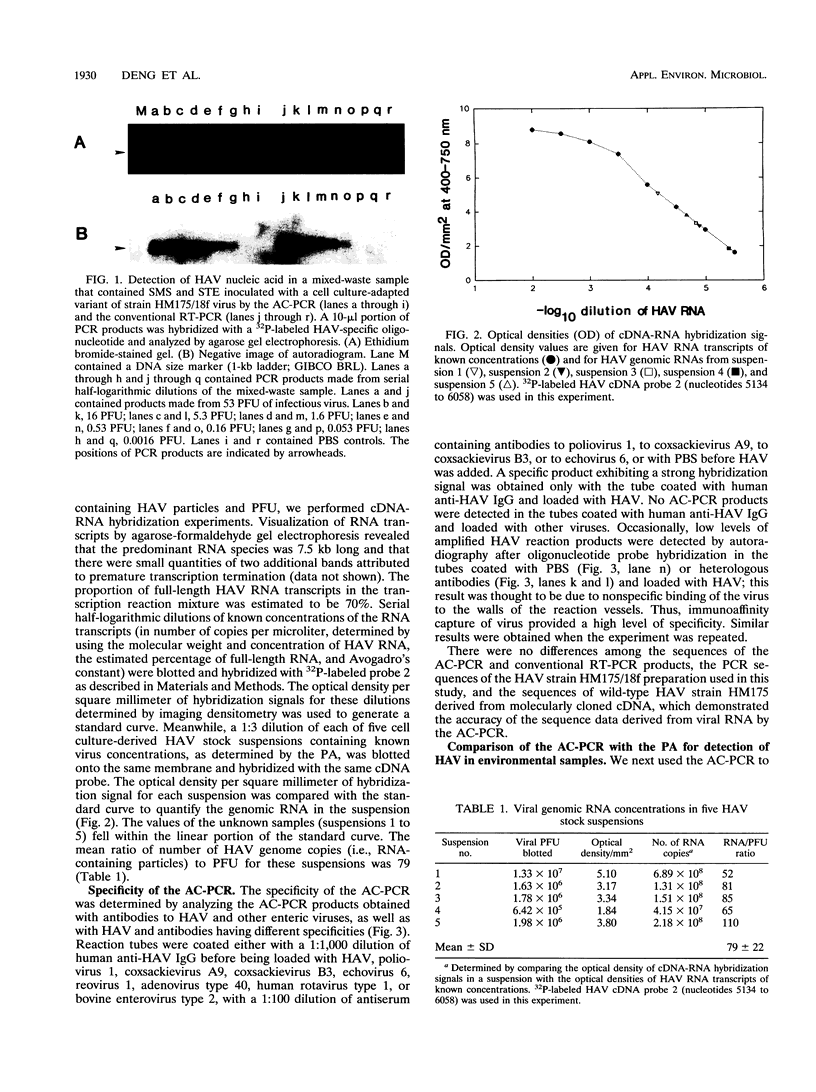
Abstract
The efficacy of the antigen-capture PCR (AC-PCR) method for the detection of hepatitis A virus (HAV) in environmental samples was demonstrated. HAV was captured from a seeded liquid waste or a shellfish sample with homologous antibody and then heat denatured and subjected to reverse transcription and the PCR, all in the same tube. Subsequently, the AC-PCR products were analyzed by oligonucleotide probe hybridization in solution, agarose gel electrophoresis, and autoradiography. The AC-PCR detected as little as 0.053 PFU of cell culture-adapted HAV strain HM175/18f. The results of cDNA-RNA hybridization indicated that the particle/PFU ratio of this virus strain is approximately 79:1. Therefore, the detection limit of the AC-PCR was estimated to be four virus particles. No amplified products were observed when poliovirus 1, coxsackievirus A9, coxsackievirus B3, echovirus 6, reovirus 1, adenovirus type 40, human rotavirus type 1, and bovine enterovirus type 2 were tested, confirming the specificity of the assay. There were no differences between the nucleotide sequences of AC-PCR products of HAV strain HM175/18f and the sequences of wild-type HAV strain HM175 derived from molecularly cloned cDNA. Of 121 waste and shellfish samples tested by both plaque assays (PA) in cell cultures and the AC-PCR, 81 (67%) were positive and 31 (26%) were negative as determined by both methods, whereas 9 (7%) were positive as determined by the AC-PCR and negative as determined by the PA, and none were positive as determined by the PA and negative as determined by the AC-PCR.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abbaszadegan M., Huber M. S., Gerba C. P., Pepper I. L. Detection of enteroviruses in groundwater with the polymerase chain reaction. Appl Environ Microbiol. 1993 May;59(5):1318–1324. doi: 10.1128/aem.59.5.1318-1324.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allard A., Girones R., Juto P., Wadell G. Polymerase chain reaction for detection of adenoviruses in stool samples. J Clin Microbiol. 1990 Dec;28(12):2659–2667. doi: 10.1128/jcm.28.12.2659-2667.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atmar R. L., Metcalf T. G., Neill F. H., Estes M. K. Detection of enteric viruses in oysters by using the polymerase chain reaction. Appl Environ Microbiol. 1993 Feb;59(2):631–635. doi: 10.1128/aem.59.2.631-635.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown V. K., Robertson B. H. Immunoselection of clinical specimens containing virus followed by polymerase chain reactor amplification and rapid direct sequencing. Biotechniques. 1990 Mar;8(3):262–264. [PubMed] [Google Scholar]
- De Leon R., Matsui S. M., Baric R. S., Herrmann J. E., Blacklow N. R., Greenberg H. B., Sobsey M. D. Detection of Norwalk virus in stool specimens by reverse transcriptase-polymerase chain reaction and nonradioactive oligoprobes. J Clin Microbiol. 1992 Dec;30(12):3151–3157. doi: 10.1128/jcm.30.12.3151-3157.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng M. Y., Cliver D. O. Inactivation of poliovirus type 1 in mixed human and swine wastes and by bacteria from swine manure. Appl Environ Microbiol. 1992 Jun;58(6):2016–2021. doi: 10.1128/aem.58.6.2016-2021.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eiden J. J., Wilde J., Firoozmand F., Yolken R. Detection of animal and human group B rotaviruses in fecal specimens by polymerase chain reaction. J Clin Microbiol. 1991 Mar;29(3):539–543. doi: 10.1128/jcm.29.3.539-543.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goswami B. B., Koch W. H., Cebula T. A. Detection of hepatitis A virus in Mercenaria mercenaria by coupled reverse transcription and polymerase chain reaction. Appl Environ Microbiol. 1993 Sep;59(9):2765–2770. doi: 10.1128/aem.59.9.2765-2770.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouvea V., Glass R. I., Woods P., Taniguchi K., Clark H. F., Forrester B., Fang Z. Y. Polymerase chain reaction amplification and typing of rotavirus nucleic acid from stool specimens. J Clin Microbiol. 1990 Feb;28(2):276–282. doi: 10.1128/jcm.28.2.276-282.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graff J., Ticehurst J., Flehmig B. Detection of hepatitis A virus in sewage sludge by antigen capture polymerase chain reaction. Appl Environ Microbiol. 1993 Oct;59(10):3165–3170. doi: 10.1128/aem.59.10.3165-3170.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen R. W., Newbold J. E., Lemon S. M. Combined immunoaffinity cDNA-RNA hybridization assay for detection of hepatitis A virus in clinical specimens. J Clin Microbiol. 1985 Dec;22(6):984–989. doi: 10.1128/jcm.22.6.984-989.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen R. W., Siegl G., Lemon S. M. Molecular epidemiology of human hepatitis A virus defined by an antigen-capture polymerase chain reaction method. Proc Natl Acad Sci U S A. 1990 Apr;87(8):2867–2871. doi: 10.1073/pnas.87.8.2867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang X., Estes M. K., Metcalf T. G., Melnick J. L. Detection of hepatitis A virus in seeded estuarine samples by hybridization with cDNA probes. Appl Environ Microbiol. 1986 Oct;52(4):711–717. doi: 10.1128/aem.52.4.711-717.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopecka H., Dubrou S., Prevot J., Marechal J., López-Pila J. M. Detection of naturally occurring enteroviruses in waters by reverse transcription, polymerase chain reaction, and hybridization. Appl Environ Microbiol. 1993 Apr;59(4):1213–1219. doi: 10.1128/aem.59.4.1213-1219.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemon S. M., Murphy P. C., Shields P. A., Ping L. H., Feinstone S. M., Cromeans T., Jansen R. W. Antigenic and genetic variation in cytopathic hepatitis A virus variants arising during persistent infection: evidence for genetic recombination. J Virol. 1991 Apr;65(4):2056–2065. doi: 10.1128/jvi.65.4.2056-2065.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotbart H. A. Enzymatic RNA amplification of the enteroviruses. J Clin Microbiol. 1990 Mar;28(3):438–442. doi: 10.1128/jcm.28.3.438-442.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shieh Y. S., Baric R. S., Sobsey M. D., Ticehurst J., Miele T. A., DeLeon R., Walter R. Detection of hepatitis A virus and other enteroviruses in water by ssRNA probes. J Virol Methods. 1991 Jan;31(1):119–136. doi: 10.1016/0166-0934(91)90150-x. [DOI] [PubMed] [Google Scholar]
- Ticehurst J. R., Feinstone S. M., Chestnut T., Tassopoulos N. C., Popper H., Purcell R. H. Detection of hepatitis A virus by extraction of viral RNA and molecular hybridization. J Clin Microbiol. 1987 Oct;25(10):1822–1829. doi: 10.1128/jcm.25.10.1822-1829.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai Y. L., Olson B. H. Detection of low numbers of bacterial cells in soils and sediments by polymerase chain reaction. Appl Environ Microbiol. 1992 Feb;58(2):754–757. doi: 10.1128/aem.58.2.754-757.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willcocks M. M., Silcock J. G., Carter M. J. Detection of Norwalk virus in the UK by the polymerase chain reaction. FEMS Microbiol Lett. 1993 Aug 15;112(1):7–12. doi: 10.1111/j.1574-6968.1993.tb06415.x. [DOI] [PubMed] [Google Scholar]
- Yang F., Xu X. A new method of RNA preparation for detection of hepatitis A virus in environmental samples by the polymerase chain reaction. J Virol Methods. 1993 Jun;43(1):77–84. doi: 10.1016/0166-0934(93)90091-5. [DOI] [PubMed] [Google Scholar]
- Yotsuyanagi H., Iino S., Koike K., Yasuda K., Hino K., Kurokawa K. Duration of viremia in human hepatitis A viral infection as determined by polymerase chain reaction. J Med Virol. 1993 May;40(1):35–38. doi: 10.1002/jmv.1890400108. [DOI] [PubMed] [Google Scholar]
- Zhou Y. J., Estes M. K., Jiang X., Metcalf T. G. Concentration and detection of hepatitis A virus and rotavirus from shellfish by hybridization tests. Appl Environ Microbiol. 1991 Oct;57(10):2963–2968. doi: 10.1128/aem.57.10.2963-2968.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]






