Abstract
Precocious puberty of cerebral origin is a poorly understood disorder of human sexual development, brought about by the premature activation of those neurons that produce luteinizing hormone-releasing hormone (LHRH), the neuropeptide controlling sexual maturation. An increased production of transforming growth factor α (TGFα) in the hypothalamus has been implicated in the mechanism underlying both normal and precocious puberty. We have now used two gene delivery systems to target TGFα overexpression near LHRH neurons in immature female rats. Fibroblasts infected with a retroviral construct in which expression of the human TGFα gene is constitutively driven by the phosphoglycerate kinase promoter, or transfected with a plasmid in which TGFα expression is controlled by an inducible metallothionein promoter, were transplanted into several regions of the hypothalamus. When the cells were in contact with LHRH nerve terminals or in the vicinity of LHRH perikarya, sexual maturation was accelerated. These results suggest that precocious puberty of cerebral origin may result from a focal disorder of TGFα production within the confines of the LHRH neuron microenvironment.
The initiation of mammalian puberty requires the activation of a group of highly specialized neurons located in the basal forebrain that produce luteinizing hormone-releasing hormone (LHRH), the decapeptide controlling sexual development and mature reproductive function (1). Hypothalamic lesions that presumably cause premature activation of LHRH neurons result in sexual precocity (2), a disorder that in human females may occur as early as the first year of life and is associated with an increased incidence of breast cancer and ovarian cysts in adulthood (3).
Most cases of female sexual precocity of central origin are diagnosed as idiopathic, as evidence for organic disfunction of the brain is found in only a minority of patients (3). Recent studies, performed in laboratory animals and nonhuman primates, have suggested that growth factors of the epidermal growth factor (EGF) family, and in particular transforming growth factor α (TGFα), produced by glial cells of the neuroendocrine brain, are involved in the developmental process that controls both normal and premature sexual maturation (4–6). Studies in rats showed that in normal puberty, TGFα expression increases mainly in the median eminence (ME) of the hypothalamus (where neuroendocrine LHRH neurons send their axons) and the preoptic-suprachiasmatic area (where many LHRH neuronal perikarya are located) (4). While blockade of EGF/TGFα receptors targeted to the ME delays puberty (4), transgenic mice overexpressing the TGFα gene under the control of a metallothionein (MT) promoter respond to activation of the promoter with LHRH release and acceleration of sexual development (7). Hypothalamic lesions that result in activation of TGFα expression in reactive astrocytes surrounding the site of injury advance puberty when located near LHRH neurons (5). These and other observations have led to the concept that activation of TGFα expression in glial cells located near LHRH neurons results in stimulation of further TGFα production from adjacent astrocytes via a paracrine route (8) and the subsequent release of neuroactive substances of glial origin able to stimulate LHRH secretion, such as prostaglandin E2 (PGE2) (9).
According to this concept, inappropriate activation of TGFα gene expression near LHRH neurons would suffice to induce female sexual precocity. To test this assumption we used the somatic cell gene therapy approach of transkaryotic implantation (10) for targeting TGFα overexpression near LHRH neurons. Two different vectors were used as carriers of the human TGFα (hTGFα) gene, a retrovirus (PGKIRES-Neo v.II) containing the constitutively active mouse phosphoglycerate kinase (PGK) promoter (M.S.-E. and X.O.B., unpublished work), and an expression plasmid (pEV142) containing the inducible mouse metallothionein-1 (MT1) promoter (11). Stable incorporation of either construct into the genome of 3T3 mouse fibroblasts endowed these cells with the ability to secrete hTGFα. While the PGK-hTGFα construct results in the continuous production of TGFα, the MT-hTGFα transgene allows one to increase TGFα synthesis at will by means of heavy metal induction of the MT promoter. Upon transplantation into different hypothalamic regions, the genetically modified cells were found to induce precocious puberty when located near LHRH cell bodies or LHRH nerve terminals, but not when placed in hypothalamic regions devoid of LHRH neurons.
MATERIALS AND METHODS
Animals.
Immature rats of the Sprague Dawley strain (Bantin and Kingman, Fremont, CA) were used (4).
Preparation of TGFα Fusion Gene Constructs.
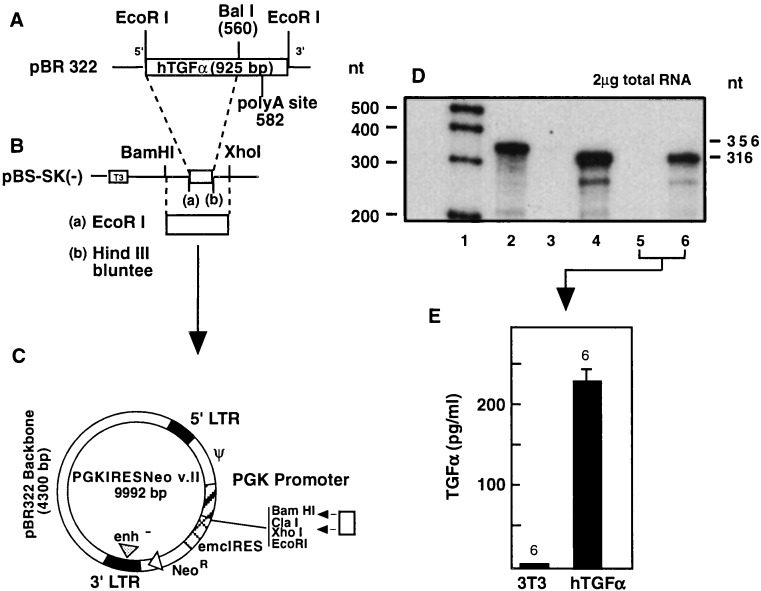
To prepare the retroviral-hTGFα construct, the entire coding region of the hTGFα gene (Fig. 1A) was first shuttled through the plasmid pBluescript-SK (Fig. 1B) and then inserted into PGKIRES-Neo v.II immediately downstream of the PGK sequence (Fig. 1C). The TGFα sequence was obtained by EcoRI digestion of plasmid phTGF1–10-925, which encodes the TGFα propeptide (provided by Graeme Bell, University of Chicago). Removal of the mRNA’s polyadenylylation signal (12) was accomplished by digestion with BalI, which recognizes a sequence immediately downstream of the TGFα termination codon.
Figure 1.
Construction of a recombinant retrovirus containing the coding region of the hTGFα gene under the control of the constitutively expressed mammalian phosphoglycerate kinase (PGK) promoter. An EcoRI–BalI cDNA fragment containing the entire coding region of hTGFα without the polyadenylylation signal (A) was subcloned into the EcoRI–HindIII sites of pBluescript (after blunting the HindIII site) (B). A BamHI–XhoI fragment was then subcloned into the multiple cloning site of PGKIRES (C) between the PGK promoter and the internal ribosomal entry site from the encephalomyocarditis virus (emcIRES), which allows efficient translation of both the hTGFα gene and the downstream neomycin-resistance gene. (D) Expression of hTGFα mRNA in transfected ψ CRIP cells and infected 3T3 cells (lane 1, DNA markers; lane 2, undigested probe; lane 3, nontransfected CRIP cells; lane 4, CRIP cells transfected with PGKIRES-hTGFα cDNA; lane 5, noninfected 3T3 cells; lane 6, 3T3 cells infected with PGKIRES-hTGFα viruses). (E) Release of TGFα from a colony of infected 3T3 cells into the culture medium.
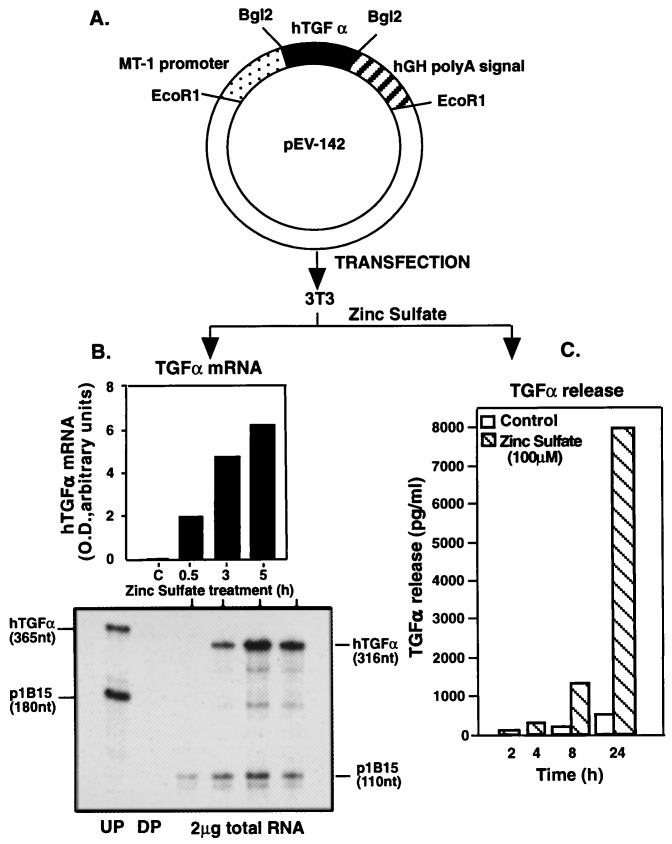
The expression plasmid pEV142, which carries the coding region of the hTGFα gene under the control of the mouse MT1 promoter (11), was a gift of G. T. Merlino (National Cancer Institute). To prepare an MT1-hTGFα antisense construct, the hTGFα cDNA from pEV-142MT1-hTGFα (11) was excised by digestion with BglII and religated to the same vector. Clones with the antisense orientation were selected by digestion with EcoRI and SphI; the latter is a unique site in the cloned hTGFα cDNA sequence.
Gene Transfer. Retroviral construct.
Replication-defective transmissible viruses were obtained by transfecting the packaging cell line psi (ψ) CRIP (13) with the PGKIRES-hTGFα plasmid, followed by selection in medium containing the neomycin analog G418 at 1 mg/ml. The cells (provided by R. C. Mulligan, Massachusetts Institute of Technology) were transfected at 50% confluency using Lipofectamine (Life Technologies).
Infection of 3T3 cells was carried out using retroviral vectors released by a colony of PGKIRES-hTGFα-expressing ψ CRIP cells. The cells were cultured in the absence of G418 for 24 h, and the virus-containing medium was collected at the end of this period, filtered through a 0.45-μm-pore filter, and added to semiconfluent 3T3 cells mixed with 160 μl of a Polybrene stock solution (100 μg/ml) in a total volume of 2 ml. The infected fibroblasts were selected for 4 weeks in G418 (1 mg/ml).
Expression of hTGFα mRNA in both ψ CRIP and 3T3 cells was detected by RNase protection assay (4), using a [32P]UTP-labeled hTGFα complementary RNA probe (11). The ability of the genetically modified fibroblasts to secrete hTGFα was assessed by radioimmunoassay (see below).
MT-hTGFα construct.
Fibroblast 3T3 cells were cotransfected as above with either an MT1-hTGFα sense or MT1-hTGFα antisense construct (2.5 μg/ml) plus pSV2-neo (0.5 μg/ml) for selection of stable transformants, using Lipofectamine. Expression of sense and antisense hTGFα mRNA transcripts was assessed by RNase protection assay using the corresponding antisense and sense complementary RNA probes. The ability of the cells to release hTGFα in response to MT1 promoter induction was assessed by measuring hTGFα in the culture medium after treating the cells with zinc sulfate (100 μM).
TGFα Radioimmunoassay.
Human TGFα released into the culture medium was measured by a highly specific radioimmunoassay (14).
Grafting.
Because of the variability in the timing of puberty that occurs between different groups of normal rats, control groups grafted with wild-type 3T3 fibroblasts were included in every experiment. In the case of cells carrying the hTGFα gene under the control of the MT1 promoter, additional controls received cells carrying the pEV142-antisense hTGFα construct. On the day of grafting, the cells were harvested by mild trypsinization (0.05% trypsin/0.5 M EDTA in PBS, pH 7.4), pelleted, and resuspended at 40,000 cells per μl in “complete” PBS (15) solution (0.1 M phosphate-buffered saline, pH 7.4, containing 1 mg/liter Ca2+, 1 mg/liter Mg2+, and 1 mg/ml glucose). The animals were positioned in a David Kopf stereotaxic instrument (incisor’s bar at +5 mm), and 1 μl of this suspension was injected on both sides of the hypothalamus using a 10 μl Hamilton microsyringe [parameters for the ME: +0.2 mm rostral from bregma, ±0.4 mm lateral from midline, −9.8 mm ventral from surface of skull; for the preoptic area (POA): +2.4 mm rostral from bregma, ±0.3 mm lateral, and −8.0 mm ventral]. Each cell suspension was injected over a 2-min period; the needle was then withdrawn 0.2 mm and was left in place for 3 more min before being slowly removed over a 1-min interval.
Heavy Metal Induction of MT1-Driven hTGFα Expression.
Starting on the day of grafting, the animals receiving grafts of 3T3 cells producing hTGFα under the control of the MT1 promoter were treated daily with zinc chloride (0.1 ml of a 50 mM solution in 0.9% saline) injected s.c. between 1300 and 1400. The injections, given at this time of the day to induce a circadian evening surge in TGFα secretion, were continued until the day of vaginal opening. Control animals receiving either nontransfected cells or cells transfected with the MT-hTGFα antisense construct were similarly treated.
Assessment of Puberty.
Starting two days after grafting (i.e., at 26 days of age) the animals were inspected daily (between 1300 and 1400) for vaginal opening. In the rat, vaginal opening usually occurs the day after the first preovulatory surge of gonadotropins (first proestrus) and represents the most overt somatic change associated with the initiation of puberty (1). To unambiguously establish the occurrence of puberty, vaginal lavages were examined daily (1300–1400) starting on the day of vaginal opening, and the type of cytology observed was used to follow the progression of the pubertal process (1). A predominance of cornified cells indicated that the animals were in the estrous phase of puberty—i.e., the presumptive day of ovulation (1). If ovulation has, in fact, occurred, vaginal lavages show 1 or 2 days later a predominance of leukocytes, reflecting the production of progesterone by the newly formed corpora lutea. All animals were, therefore, killed on the day of first diestrus following the first estrus, and the presence of corpora lutea in the ovaries was confirmed by visual inspection. In most cases, the first diestrus occurred the day after vaginal opening—i.e., after 1 day in estrus. Less than 10% of the animals showed 2 estrous days after vaginal opening, but like the rest of the animals, these rats were also killed on the day of first diestrus.
In Situ Detection of Grafted Cells.
Once the animals reached puberty (as defined above), their brains were fixed and processed for hybridization histochemistry, as described (4). LHRH neurons and their processes were detected by immunohistochemistry in 30-μm sections using HU4H, a highly specific monoclonal antibody (16) at a 1:3000 dilution, as described (17). Upon completion of this reaction, which was performed on floating sections, the sections were mounted on glass slides and dried overnight under vacuum. The next morning they were hybridized to the same complementary RNA probe used to detect hTGFα mRNA in RNase protection assays, but labeled with digoxigenin-UTP. The hybridization procedure employed has been described in detail elsewhere (18).
Detection of grafted cells in the vicinity of LHRH nerve fibers or cell bodies (or away from them) was a straightforward procedure due to the clearcut differences in color between the LHRH immunohistochemical reaction (brown) and the hTGFα mRNA hybridization (purple). Control 3T3 cells bearing the MT-hTGFα construct with the hTGFα gene in the antisense orientation were identified using a sense RNA probe. Control grafts of wild-type 3T3 cells could not, however, be so accurately localized because of the lack of a specific marker. Localization in this case relied on identifying the tracks of the needle.
Statistics.
The data were evaluated by using a one-way analysis of variance followed by the Student–Neuman–Keuls multiple-range test for individual means.
RESULTS
RNase protection assay demonstrated the expression of human TGFα mRNA in G418-resistant ψ CRIP colonies. Fig. 1D shows the mRNA levels detected in one of these colonies. Although having a low titer (300 colony-forming units/ml), retrovirus vectors released by PGKIRES-hTGFα-transfected ψ CRIP cells infected 3T3 fibroblasts, as evidenced by the presence of hTGFα mRNA in these cells after G418 selection (Fig. 1D) and the constitutive release of TGFα protein to the incubation medium (Fig. 1E).
3T3 fibroblasts that stably incorporated the pEV142-hTGFα cassette (Fig. 2A) responded to zinc sulfate with both an increase in hTGFα mRNA content (Fig. 2B) and TGFα protein release (Fig. 2C). While a marked increase in hTGFα mRNA content was observed after 5 h of treatment, maximal peptide accumulation occurred at 24 h. Fig. 2 B and C depicts the colony which had the highest TGFα production in response to zinc, and was, therefore, selected for transplantation. 3T3 cells carrying the hTGFα cDNA in the antisense orientation also responded to zinc sulfate with increased levels of antisense hTGFα mRNA transcripts (not shown).
Figure 2.
Zinc-induced activation of hTGFα production by 3T3 cells bearing a fusion gene (A) consisting of the hTGFα gene under the control of the mouse MT1 promoter (11). (B) Changes in hTGFα mRNA abundance in a transfected colony after treatment with zinc sulfate (100 μM), as determined by RNase protection assay. UP, undigested probe; DP, digested probe. (C) Release of TGFα protein to the culture medium in response to zinc by the colony depicted in B.
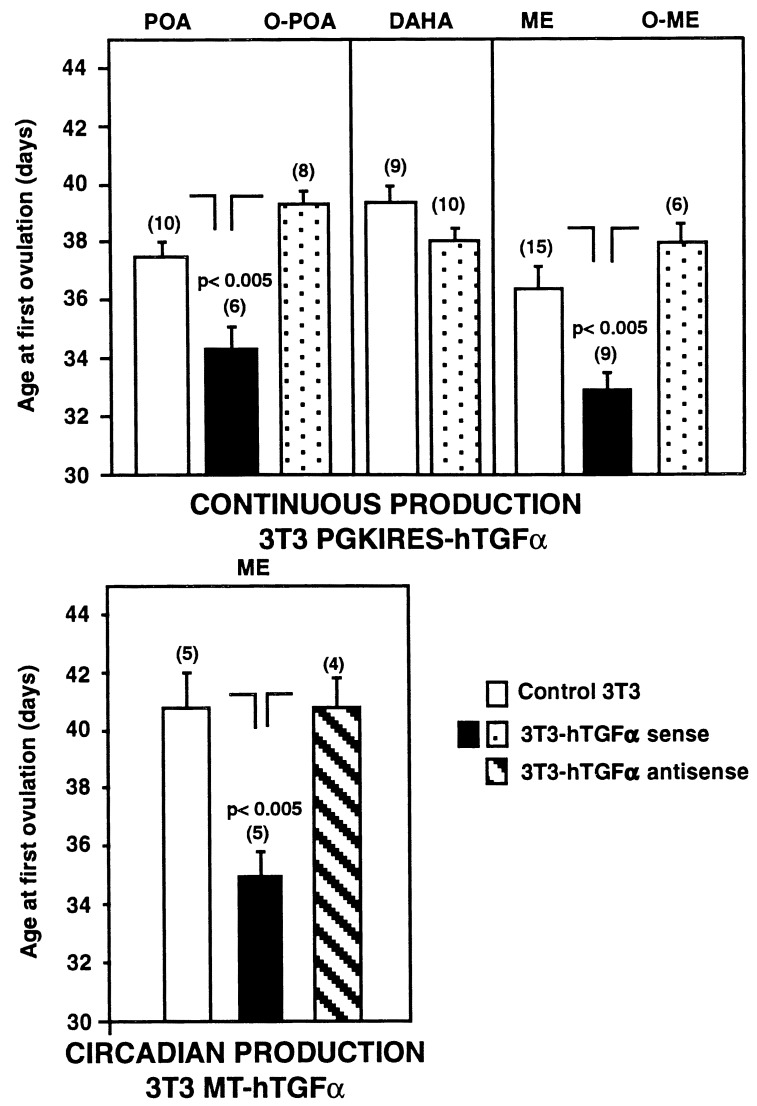
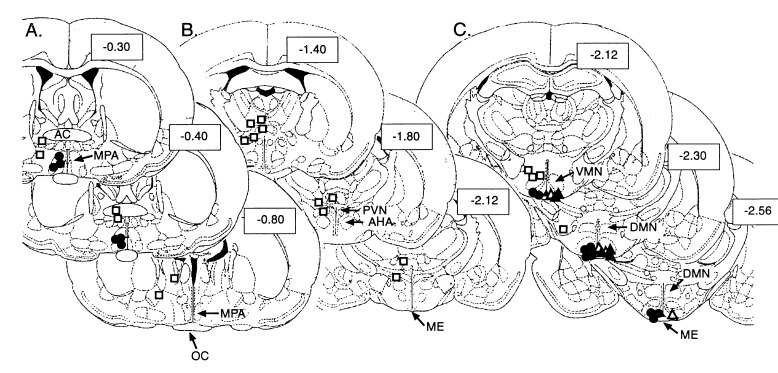
As expected, there was variability in the timing of puberty among different groups of control 3T3-grafted rats (compare, for instance, the left and right panels of Fig. 4 Upper). Puberty was, however, clearly advanced in animals receiving intrahypothalamic grafts of TGFα-producing cells in comparison with their contemporary 3T3-grafted controls. Grafting cells infected with PGKIRES-hTGFα (continuous production of hTGFα) in different hypothalamic regions demonstrated that puberty was advanced only when the cells were located in the immediate vicinity of LHRH neuronal perikarya in the POA of the hypothalamus or adjacent to LHRH nerve terminals in the ME. Misplaced implants or cells transplanted dorsal to the anterior hypothalamic area (DAHA), i.e., to a region devoid of LHRH neurons, were ineffective. Fig. 3 depicts the anatomical localization of the grafts in each animal; Fig. 4 presents the statistical analysis of the results; and Fig. 5 provides examples of hTGFα mRNA-expressing cells grafted in the vicinity of LHRH perikarya within the POA (Fig. 5G), or in contact with the LHRH nerve terminals of the ME (Fig. 5 B and E). Grafts located within the hypothalamic tissue itself had the circumscribed aspect (see, for instance, Fig. 5G) typically acquired by fibroblastic cells transplanted into the brain (20). In contrast, cells transplanted into the ME spread along the external surface of the ME, presumably due to the ventral location of the injection sites. Animals bearing grafts of control 3T3 cells in either the POA and ME ovulated slightly earlier than animals with misplaced PGKIRES-hTGFα transplants (Fig. 4). This is probably due to an astrocytic reaction in response to the small lesion caused by the microinjection, as larger lesions of the hypothalamus that cause activation of TGFα expression in reactive astrocytes surrounding the lesion site have been shown to accelerate sexual maturation (5). Misplaced PGKIRES-hTGFα transplants failed to affect sexual development (Fig. 4); six of them were found lateral to the POA (Fig. 3A). Another four intended for the ME were found either lateral to the ME (one graft) or between the ventromedial and dorsomedial nuclei of the hypothalamus (three grafts) (Fig. 3C). In addition, four grafts (two intended for the POA and two intended for the ME) could not be localized by the in situ hybridization procedure used to detect the hTGFα gene. These cells may have prematurely lost the ability to produce hTGFα after transplantation due to an early immunological rejection or to inactivation of the PGK promoter in a retroviral context (15). Alternatively, the site of injection may have been too ventral (or within the third ventricle), allowing the cells to be removed by cerebrospinal fluid. Rats bearing grafts that could not be localized were included in the groups of misplaced implants (O-POA and O-ME in Fig. 4).
Figure 4.
Advancement of female puberty by the intrahypothalamic transplantation of cells genetically engineered to overexpress the TGFα gene. (Upper) Effect on puberty of the continuous production of TGFα after transplantation of cells bearing a recombinant PGKIRES-hTGFα retroviral construct in which expression of the TGFα gene is driven by a constitutively expressed promoter. (Lower) Transplantation of cells in which TGFα expression is under the control of an inducible promoter advances sexual maturation when the cells are implanted in the ME region of the hypothalamus, and expression of the TGFα gene is induced by daily injections of zinc chloride. O-POA, outside the POA; DAHA, region dorsal to the anterior hypothalamic area; O-ME, outside the ME. Numbers on top of bars are number of animals per group. Vertical lines are SEM. The O-POA and O-ME groups of PGKIRES-hTGFα-implanted rats each include two animals in which the implants could not be localized (see text).
Figure 3.
Diagrammatic representation of the hypothalamic sites receiving grafts of hTGFα-producing 3T3 cells. Although the cells were injected bilaterally, only one site is shown for purposes of clarity. In spite of the intrinsic variability of stereotaxic surgery, the location of contralateral implants was, for the most part, consistent with the depicted sites. The location of control grafts (3T3 cells), though not represented, overlapped with that shown for hTGFα-producing cells. Each series depicts three coronal planes of the suprachiasmatic region (A), anterior hypothalamus (B), and medial basal hypothalamus (C), respectively. The sections outlined correspond to those described in Paxinos and Watson’s atlas of the rat brain (19). The numbers inside boxes represent distance from bregma in millimeters. • and ▴, sites effective in accelerating the onset of puberty; •, PGKIRES-hTGFα; ▴, MT-hTGFα; □, PGKIRES-hTGFα ineffective sites; and ▵, MT-antisense hTGFα. The PGKIRES-hTGFα grafts could not be found in two animals in which the intended site was the POA and two rats in which the intended site was the ME. There were no misplaced MT-hTGFα implants. AC, anterior commissura; AHA, anterior hypothalamic area; DMN, dorsomedial nucleus; ME, median eminence; MPA, medial preoptic area; OC, optic chiasm; PVN, paraventricular nucleus; VMN, ventromedial nucleus.
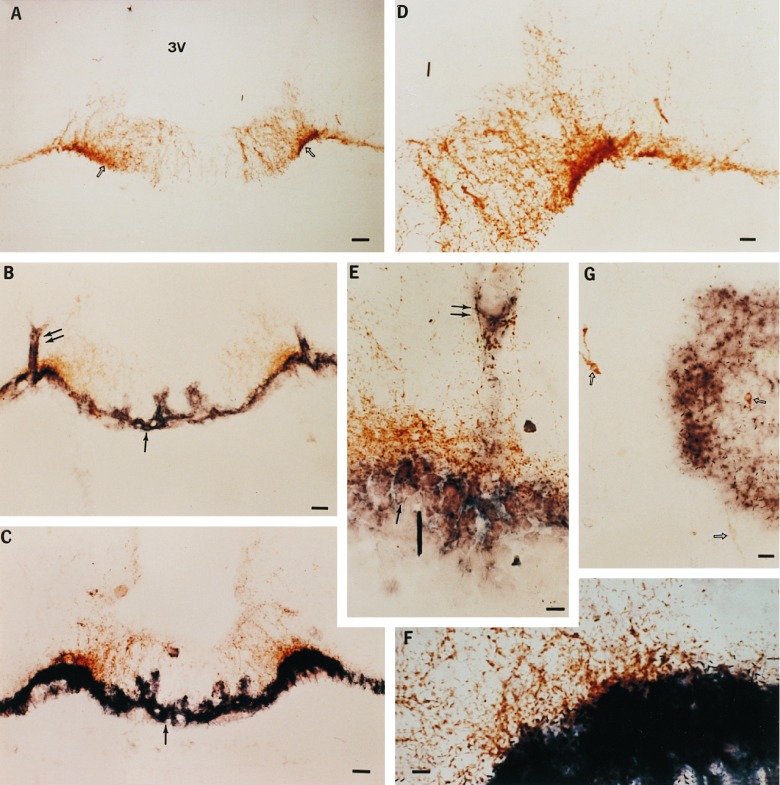
Figure 5.
Localization of TGFα-producing cells transplanted into the hypothalamus of immature female rats by combined immunohistochemistry/in situ hybridization. (A) Low-magnification view (×77) of LHRH nerve terminals (open arrows) in the ME of an animal that received a transplant of control 3T3 cells. 3V, third ventricle. (B) Low-magnification view (×77) of the ME of an animal that underwent precocious puberty after receiving a transplant of TGFα-producing 3T3 cells. In this case TGFα production is continuous, as the TGFα gene inserted into the PGKIRES retrovirus is driven by the constitutively activated PGK promoter. The double arrows denote the sites of microinjection, and the single arrow points to the transplanted cells identified by their content of hTGFα. hTGFα mRNA was detected by hybridization to a digoxigenin-UTP-labeled complementary RNA probe. (C) Low-magnification view (×77) of the ME of an animal that underwent precocious puberty after receiving a transplant of 3T3 cells bearing the hTGFα gene under the control of mouse MT1 inducible promoter. The animal was perfused 5 h after receiving a single subcutaneous injection of zinc chloride to activate the MT promoter. (D, E, and F) Higher-magnification views of A, B, and C, respectively. (D, ×140; E and F, ×420.) (G) View (×140) of the POA of an animal that showed advanced puberty after receiving a transplant of 3T3 cells bearing the PGKIRES-hTGFα retroviral construct into the POA. Notice that the hTGFα-positive cells are in close proximity to a group of LHRH neurons (arrows). (Bars: A, B, and C = 50 μm; D and G = 25 μm; E and F = 10 μm.)
Implantation of pEV142-hTGFα-containing cells into the ME (Fig. 3C), followed by daily injections of zinc chloride to produce a circadian increase in TGFα production, resulted in a marked advancement of puberty compared with animals receiving a transplant of either nontransfected 3T3 cells or cells carrying the MT-antisense hTGFα construct (Fig. 4 Lower). As in the case of PGKIRES, cells carrying pEV142-hTGFα were found in the ventral aspect of the ME in close apposition to LHRH nerve terminals (Fig. 5 C and F); some cells appeared to have penetrated into the ME itself.
DISCUSSION
The present study demonstrates that a circumscribed increase in the production of TGFα within the neuroendocrine brain suffices to set in motion the cascade of events leading to the acquisition of female sexual maturity. The results also make clear that for precocious puberty to occur, the increased production of TGFα must occur in the vicinity of either the LHRH nerve terminals or the LHRH perikarya. The effectiveness of TGFα-producing cells in accelerating puberty when transplanted into the median eminence of the hypothalamus—i.e., adjacent to LHRH nerve terminals—supports the contention that the ME is a major site of the normal interaction between TGFα-producing cells and the LHRH neuronal network (4, 9). The results also show that puberty can be advanced by either a continuous (PKG-hTGFα) or circadian (MT-hTGFα) increase in TGFα production. Although it is clear that a single injection of zinc chloride potently activates the MT promoter in vivo (7, 11), we do not know the precise kinetics of TGFα release in response to the injection. Under in vitro conditions maximal accumulation of the peptide was observed after 24 h of continuous exposure to zinc (Fig. 2), but no such accumulation would be expected to occur in vivo because of ongoing metabolism and removal of the peptide from its site of production. An additional factor that may temporally restrict TGFα release from the grafted cells in response to zinc is down-regulation of gene expression. Immunohistochemical examination of one animal 24 h after the last zinc chloride injection revealed a weak hTGFα hybridization signal in the transplanted cells, suggesting a decrease in gene expression. Down-regulation of hTGFα gene expression was, in fact, also observed in vitro after 24 h of exposure to zinc sulfate (not shown).
TGFα initiates its biological actions by binding to the EGF receptor (EGFR). The ability of human TGFα to activate rodent EGFR has been previously shown in transgenic mice carrying the same MT-hTGFα transgene employed in the present experiments (11). Although ligand-dependent activation of EGFR initiates a complex signaling cascade (21), an event directly relevant to the control of LHRH release is the induction of cyclooxygenase (22), the enzyme that catalyzes the metabolism of arachidonic acid to prostaglandins. A previous study demonstrated the ability of TGFα to stimulate the release of PGE2, a potent LHRH secretagogue, from the ME (9), making it likely that TGFα released by the grafts accelerates the onset of puberty by the same cell–cell communication mechanism.
Since functional EGFRs can be detected in hypothalamic astrocytes (8, 23), but not in LHRH neurons (17, 23), we have postulated that TGFα produced by astrocytes acts in a paracrine manner to stimulate the glial secretion of neuroactive substances able to stimulate LHRH release. More recently, we identified PGE2 as one of these substances (24). It is thus plausible that the process by which hTGFα-producing grafts accelerate puberty is by stimulating PGE2 release from adjacent astrocytes. Release of PGE2 from the grafted cells themselves in response to hTGFα may represent an additional source of the prostaglandin. 3T3 cells have been shown to respond to EGF with increased metabolism of arachidonic acid to PGE2 and PGE2α (25).
In a more general sense, and considering that glial astrocytes represent a significant source of TGFα in the hypothalamus (4, 8), the present results highlight the relevance of growth factor-mediated glial–neuronal communication in the central process that controls the onset of mammalian puberty. It is now clear that astrocytes can affect neuronal function by means of release of a variety of neuroactive substances (26–28). We have previously provided evidence for the concept that not only TGFα (4, 9) but also neuregulins,** a family of TGFα relatives (29), are involved in the glial–neuronal process that controls LHRH neuronal function. By demonstrating the need of anatomical proximity between TGFα-producing cells and LHRH neurons, the present results emphasize the importance of this mechanism of glial–neuronal communication. Importantly, they also suggest that a focal derangement of this communication might contribute to both the etiology of idiopathic precocious puberty of central origin and to the mechanisms by which organic lesions of the hypothalamus hasten sexual maturation in humans. In the former case, discrete alterations of glial activity near LHRH neurons may stimulate LHRH secretion and thus result in sexual precocity, without causing a detectable organic lesion. In the case of hypothalamic lesions, if they are in the vicinity of LHRH neurons, the attendant changes in glial activity would provide the necessary stimulation for LHRH release to increase. If the lesion is caused by tumors, the tumor itself may be able to turn on the glial–neuronal signaling mechanism that enhances LHRH secretion. This idea is supported by our recent finding of TGFα-producing astrocytes in a hypothalamic hamartoma removed from a 2-year-old girl with sexual precocity (H. Jung, P. Carmel, J. Watkins, and S.R.O., unpublished results). Although the effectiveness of hypothalamic hamartomas in causing sexual precocity (3) has been attributed to the presence of ectopic LHRH neurons in the tumor (30), our findings indicate that production of growth factors able to enhance LHRH release from the normal LHRH neuronal network may also play an important role. In fact, the hamartoma in which we detected TGFα-producing cells was devoid of LHRH neurons.
The advancement of puberty induced by the TGFα-producing grafts was clear-cut, but it did not occur immediately after grafting, suggesting that manifestation of the TGFα effect requires maturation of additional components of the molecular machinery controlling LHRH neuronal activity. This temporal delay is similar to that observed when precocious puberty is induced by activation of the excitatory transsynaptic control of LHRH neurons, by means of stimulation of N-methyl-d-aspartate receptors (31, 32). The fact that, in human females, sexual precocity may be manifested as early as during the first year of life—i.e., during infantile development—suggests the existence of upstream regulatory components responsible for the coordinated activation of the transsynaptic and glial input to the LHRH neuronal network. Even though these hypothetical factors are yet to be identified, the present results demonstrate that the site-specific activation of a single regulatory component, such as TGFα, suffices to accelerate significantly the overall process of female sexual development. The effectiveness and regional specificity of the effect indicates that TGFα is an integral part of the physiological process that controls the initiation of mammalian puberty.
Acknowledgments
This work was supported by National Institutes of Health Grants HD25123 (S.R.O.), P30 Population Center Grant HD18185, RR00163 for the operation of the Oregon Regional Primate Research Center, NS-24279 (X.O.B.), and Ca-46413 (R.J.C.). R.J.C. is a Veterans Association Clinical Investigator.
ABBREVIATIONS
- LHRH
luteinizing hormone-releasing hormone
- TGFα
transforming growth factor α
- hTGFα
human TGFα
- EGF
epidermal growth factor
- EGFR
EGF receptor
- PGE2
prostaglandin E2
- ME
median eminence
- POA
preoptic area
- PGK
phosphoglycerate kinase
- MT
metallothionein
Footnotes
Ma, Y. J., Berg-von der Emde, K., Hill, D. F., Costa, M. E. & Ojeda, S. R., Proceedings of the 10th International Congress of Endocrinology, June 12–15, 1996, San Francisco, CA, p. 220.
References
- 1.Ojeda S R, Urbanski H F. In: The Physiology of Reproduction. 2nd Ed. Knobil E, Neill J D, editors. Vol. 2. New York: Raven; 1994. pp. 363–409. [Google Scholar]
- 2.Donovan B T, van der Werff ten Bosch J J. Nature (London) 1956;178:745. doi: 10.1038/178745a0. [DOI] [PubMed] [Google Scholar]
- 3.Grumbach M M, Styne D M. In: Williams Textbook of Endocrinology. 8th Ed. Wilson J D, Foster D W, editors. Philadelphia: Saunders; 1992. pp. 1139–1221. [Google Scholar]
- 4.Ma Y J, Junier M, Costa M E, Ojeda S R. Neuron. 1992;9:657–670. doi: 10.1016/0896-6273(92)90029-d. [DOI] [PubMed] [Google Scholar]
- 5.Junier M, Ma Y J, Costa M E, Hoffman G, Hill D F, Ojeda S R. Proc Natl Acad Sci USA. 1991;88:9743–9747. doi: 10.1073/pnas.88.21.9743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ma Y J, Costa M E, Ojeda S R. Neuroendocrinology. 1994;60:346–359. doi: 10.1159/000126769. [DOI] [PubMed] [Google Scholar]
- 7.Ma Y J, Dissen G A, Merlino G, Coquelin A, Ojeda S R. Endocrinology. 1994;135:1392–1400. doi: 10.1210/endo.135.4.7925101. [DOI] [PubMed] [Google Scholar]
- 8.Ma Y J, Berg-von der Emde K, Moholt-Siebert M, Hill D F, Ojeda S R. J Neurosci. 1994;14:5644–5651. doi: 10.1523/JNEUROSCI.14-09-05644.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ojeda S R, Urbanski H F, Costa M E, Hill D F, Moholt-Siebert M. Proc Natl Acad Sci USA. 1990;87:9698–9702. doi: 10.1073/pnas.87.24.9698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Selden R F, Skoskiewicz M J, Howie K B, Russell P S, Goodman H M. Science. 1987;236:714–718. doi: 10.1126/science.3472348. [DOI] [PubMed] [Google Scholar]
- 11.Jhappan C, Stahle C, Harkins R N, Fausto N, Smith G H, Merlino G T. Cell. 1990;61:1137–1146. doi: 10.1016/0092-8674(90)90076-q. [DOI] [PubMed] [Google Scholar]
- 12.Valerio D. In: Transgenic Animals. Grosveld F, Kollias G, editors. San Diego: Academic; 1992. pp. 211–239. [Google Scholar]
- 13.Danos O, Mulligan R C. Proc Natl Acad Sci USA. 1988;85:6460–6464. doi: 10.1073/pnas.85.17.6460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Halter S A, Dempsey P, Matsui Y, Stokes M K, Graves-Deal R, Hogan B L M, Coffey R J. Am J Pathol. 1992;140:1131–1146. [PMC free article] [PubMed] [Google Scholar]
- 15.Frim D M, Short M P, Rosenberg W S, Simpson J, Breakefield X O, Isacson O. J Neurosurg. 1993;78:267–273. doi: 10.3171/jns.1993.78.2.0267. [DOI] [PubMed] [Google Scholar]
- 16.Urbanski H F. Biol Reprod. 1991;44:681–686. doi: 10.1095/biolreprod44.4.681. [DOI] [PubMed] [Google Scholar]
- 17.Ma Y J, Hill D F, Junier M, Costa M E, Felder S E, Ojeda S R. Mol Cell Neurosci. 1994;5:246–262. doi: 10.1006/mcne.1994.1029. [DOI] [PubMed] [Google Scholar]
- 18.Berg-von der Emde K, Dees W L, Hiney J K, Hill D F, Dissen G A, Costa M E, Moholt-Siebert M, Ojeda S R. J Neurosci. 1995;15:4223–4237. doi: 10.1523/JNEUROSCI.15-06-04223.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 2nd Ed. New York: Academic; 1986. [DOI] [PubMed] [Google Scholar]
- 20.Gage F H, Kawaja M D, Fisher L J. Trends Neurosci. 1991;14:328–333. doi: 10.1016/0166-2236(91)90156-o. [DOI] [PubMed] [Google Scholar]
- 21.Schlessinger J, Ullrich A. Neuron. 1992;9:383–391. doi: 10.1016/0896-6273(92)90177-f. [DOI] [PubMed] [Google Scholar]
- 22.Peppelenbosch M P, Tertoolen L G J, Hage W J, de Laat S W. Cell. 1993;74:565–575. doi: 10.1016/0092-8674(93)80057-l. [DOI] [PubMed] [Google Scholar]
- 23.Voigt P, Ma Y J, Gonzalez D, Fahrenbach W H, Wetsel W C, Berg-von der Emde K, Hill D F, Taylor K G, Costa M E, Seidah N G, Ojeda S R. Endocrinology. 1996;137:2593–2605. doi: 10.1210/endo.137.6.8641214. [DOI] [PubMed] [Google Scholar]
- 24.Ma Y J, Berg-von der Emde K, Rage F, Wetsel W C, Ojeda S R. Endocrinology. 1997;138:19–25. doi: 10.1210/endo.138.1.4863. [DOI] [PubMed] [Google Scholar]
- 25.Nolan R D, Danilowicz R M, Eling T E. Mol Pharmacol. 1988;33:650–656. [PubMed] [Google Scholar]
- 26.Barres B A. J Neurosci. 1991;11:3685–3694. doi: 10.1523/JNEUROSCI.11-12-03685.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Martin D L. Glia. 1992;5:81–94. doi: 10.1002/glia.440050202. [DOI] [PubMed] [Google Scholar]
- 28.Garcia-Segura L M, Chowen J A, Naftolin F. Front Neuroendocrinol. 1996;17:180–211. doi: 10.1006/frne.1996.0005. [DOI] [PubMed] [Google Scholar]
- 29.Wen D, Peles E, Cupples R, Suggs S V, Bacus S S, Luo Y, Trail G, Hu S, Silbiger S M, Ben Levy R, Koski R A, Lu H S, Yarden Y. Cell. 1992;69:559–572. doi: 10.1016/0092-8674(92)90456-m. [DOI] [PubMed] [Google Scholar]
- 30.Judge D M, Kulin H E, Page R, Santen R, Trapukda S. N Engl J Med. 1977;296:7–10. doi: 10.1056/NEJM197701062960102. [DOI] [PubMed] [Google Scholar]
- 31.Urbanski H F, Ojeda S R. Neuroendocrinology. 1987;46:273–276. doi: 10.1159/000124831. [DOI] [PubMed] [Google Scholar]
- 32.Plant T M, Gay V L, Marshall G R, Arslan M. Proc Natl Acad Sci USA. 1989;86:2506–2510. doi: 10.1073/pnas.86.7.2506. [DOI] [PMC free article] [PubMed] [Google Scholar]