Abstract
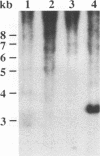
The aroA gene of Pasteurella haemolytica serotype A1 was cloned by complementation of the aroA mutation in Escherichia coli K-12 strain AB2829. The nucleotide sequence of a 2.2-kb fragment encoding aroA predicted an open reading frame product 434 amino acids long that shows homology to other bacterial AroA proteins. Several strategies to inactivate aroA were unsuccessful. Gene replacement was finally achieved by constructing a replacement plasmid with aroA inactivated by insertion of a P. haemolytica ampicillin resistance fragment into a unique NdeI site in aroA. A hybrid plasmid was constructed by joining the aroA replacement plasmid with a 4.2-kb P. haemolytica plasmid which encodes streptomycin resistance. Following PhaI methylation, the replacement plasmid was introduced by electroporation into P. haemolytica NADC-D60, a plasmidless strain of serotype 1A. Allelic exchange between the replacement plasmid and the chromosome of P. haemolytica gave rise to an ampicillin-resistant mutant which grew on chemically defined P. haemolytica medium supplemented with aromatic amino acids but failed to grow on the same medium lacking tryptophan. Southern blot analysis confirmed that aroA of the mutant was inactivated and that the mutant was without a plasmid.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Azad A. K., Coote J. G., Parton R. Distinct plasmid profiles of Pasteurella haemolytica serotypes and the characterization and amplification in Escherichia coli of ampicillin-resistance plasmids encoding ROB-1 beta-lactamase. J Gen Microbiol. 1992 Jun;138(6):1185–1196. doi: 10.1099/00221287-138-6-1185. [DOI] [PubMed] [Google Scholar]
- BACON G. A., BURROWS T. W., YATES M. The effects of biochemical mutation on the virulence on Bacterium typhosum: the induction and isolation of mutants. Br J Exp Pathol. 1950 Dec;31(6):703–713. [PMC free article] [PubMed] [Google Scholar]
- Bowe F., O'Gaora P., Maskell D., Cafferkey M., Dougan G. Virulence, persistence, and immunogenicity of Yersinia enterocolitica O:8 aroA mutants. Infect Immun. 1989 Oct;57(10):3234–3236. doi: 10.1128/iai.57.10.3234-3236.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briggs R. E., Tatum F. M., Casey T. A., Frank G. H. Characterization of a restriction endonuclease, PhaI, from Pasteurella haemolytica serotype A1 and protection of heterologous DNA by a cloned PhaI methyltransferase gene. Appl Environ Microbiol. 1994 Jun;60(6):2006–2010. doi: 10.1128/aem.60.6.2006-2010.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang Y. F., Ma D. P., Bai H. Q., Young R., Struck D. K., Shin S. J., Lein D. H. Characterization of plasmids with antimicrobial resistant genes in Pasteurella haemolytica A1. DNA Seq. 1992;3(2):89–97. doi: 10.3109/10425179209034001. [DOI] [PubMed] [Google Scholar]
- Collins F. M. Vaccines and cell-mediated immunity. Bacteriol Rev. 1974 Dec;38(4):371–402. doi: 10.1128/br.38.4.371-402.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan K., Coggins J. R. The serC-aro A operon of Escherichia coli. A mixed function operon encoding enzymes from two different amino acid biosynthetic pathways. Biochem J. 1986 Feb 15;234(1):49–57. doi: 10.1042/bj2340049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman A. M., Long S. R., Brown S. E., Buikema W. J., Ausubel F. M. Construction of a broad host range cosmid cloning vector and its use in the genetic analysis of Rhizobium mutants. Gene. 1982 Jun;18(3):289–296. doi: 10.1016/0378-1119(82)90167-6. [DOI] [PubMed] [Google Scholar]
- Hansen E. J., Latimer J. L., Thomas S. E., Helminen M., Albritton W. L., Radolf J. D. Use of electroporation to construct isogenic mutants of Haemophilus ducreyi. J Bacteriol. 1992 Aug;174(16):5442–5449. doi: 10.1128/jb.174.16.5442-5449.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoiseth S. K., Stocker B. A. Aromatic-dependent Salmonella typhimurium are non-virulent and effective as live vaccines. Nature. 1981 May 21;291(5812):238–239. doi: 10.1038/291238a0. [DOI] [PubMed] [Google Scholar]
- Hoiseth S. K., Stocker B. A. Genes aroA and serC of Salmonella typhimurium constitute an operon. J Bacteriol. 1985 Jul;163(1):355–361. doi: 10.1128/jb.163.1.355-361.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homchampa P., Strugnell R. A., Adler B. Molecular analysis of the aroA gene of Pasteurella multocida and vaccine potential of a constructed aroA mutant. Mol Microbiol. 1992 Dec;6(23):3585–3593. doi: 10.1111/j.1365-2958.1992.tb01794.x. [DOI] [PubMed] [Google Scholar]
- Livrelli V., Peduzzi J., Joly B. Sequence and molecular characterization of the ROB-1 beta-lactamase gene from Pasteurella haemolytica. Antimicrob Agents Chemother. 1991 Feb;35(2):242–251. doi: 10.1128/aac.35.2.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micheletti P. A., Sment K. A., Konisky J. Isolation of a coenzyme M-auxotrophic mutant and transformation by electroporation in Methanococcus voltae. J Bacteriol. 1991 Jun;173(11):3414–3418. doi: 10.1128/jb.173.11.3414-3418.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Gaora P., Maskel D., Coleman D., Cafferkey M., Dougan G. Cloning and characterisation of the serC and aroA genes of Yersinia enterocolitica, and construction of an aroA mutant. Gene. 1989 Dec 7;84(1):23–30. doi: 10.1016/0378-1119(89)90135-2. [DOI] [PubMed] [Google Scholar]
- Pittard J., Wallace B. J. Distribution and function of genes concerned with aromatic biosynthesis in Escherichia coli. J Bacteriol. 1966 Apr;91(4):1494–1508. doi: 10.1128/jb.91.4.1494-1508.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts M., Maskell D., Novotny P., Dougan G. Construction and characterization in vivo of Bordetella pertussis aroA mutants. Infect Immun. 1990 Mar;58(3):732–739. doi: 10.1128/iai.58.3.732-739.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sost D., Amrhein N. Substitution of Gly-96 to Ala in the 5-enolpyruvylshikimate-3-phosphate synthase of Klebsiella pneumoniae results in a greatly reduced affinity for the herbicide glyphosate. Arch Biochem Biophys. 1990 Nov 1;282(2):433–436. doi: 10.1016/0003-9861(90)90140-t. [DOI] [PubMed] [Google Scholar]
- Verma N. K., Lindberg A. A. Construction of aromatic dependent Shigella flexneri 2a live vaccine candidate strains: deletion mutations in the aroA and the aroD genes. Vaccine. 1991 Jan;9(1):6–9. doi: 10.1016/0264-410x(91)90308-s. [DOI] [PubMed] [Google Scholar]
- Wessman G. E. Cultivation of Pasteurella haemolytica in a chemically defined medium. Appl Microbiol. 1966 Jul;14(4):597–602. doi: 10.1128/am.14.4.597-602.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zealey G. R., Loosmore S. M., Yacoob R. K., Cockle S. A., Boux L. J., Miller L. D., Klein M. H. Gene replacement in Bordetella pertussis by transformation with linear DNA. Biotechnology (N Y) 1990 Nov;8(11):1025–1029. doi: 10.1038/nbt1190-1025. [DOI] [PubMed] [Google Scholar]