Abstract
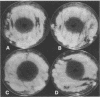
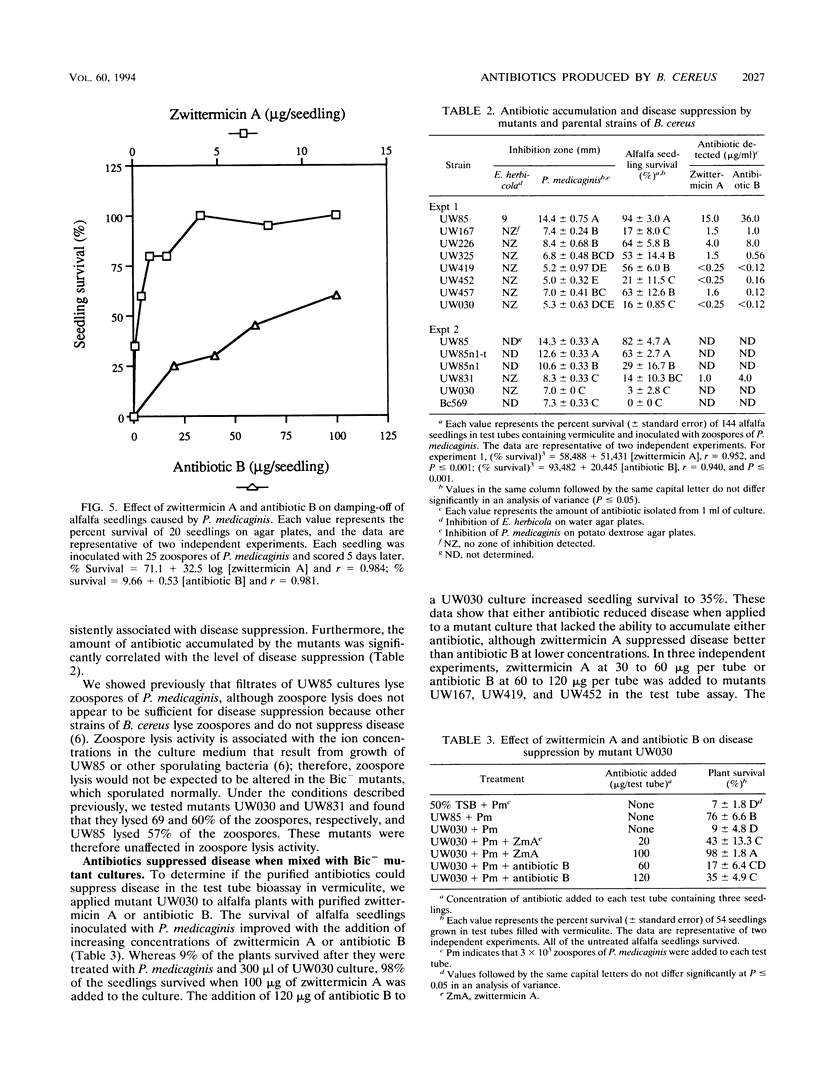
Cultures and culture filtrates of Bacillus cereus UW85 suppress damping-off of alfalfa caused by Phytophthora medicaginis. We studied the role in disease suppression of two antibiotics from culture filtrates of UW85 that reversibly inhibited growth of P. medicaginis. We purified the two antibiotics by cation-exchange chromatography and high-voltage paper electrophoresis and showed that one of them, designated zwittermicin A, was an aminopolyol of 396 Da that was cationic at pH 7.0; the second, designated antibiotic B, appeared to be an aminoglycoside containing a disaccharide. Both antibiotics prevented disease of alfalfa seedlings caused by P. medicaginis. Purified zwittermicin A reversibly reduced elongation of germ tubes derived from cysts of P. medicaginis, and antibiotic B caused swelling of the germ tubes. Mutants generated with Tn917 or mitomycin C treatment were screened either for antibiotic accumulation in an agar plate diffusion assay or for the ability to suppress damping-off disease of alfalfa. Of 2,682 mutants screened for antibiotic accumulation, 5 mutants were substantially reduced in antibiotic accumulation and disease-suppressive activity. Of the 1,700 mutants screened for disease-suppressive activity, 3 mutants had reduced activity and they accumulated less of both antibiotics than did the parent strain. The amount of antibiotic accumulated by the mutants was significantly correlated with the level of disease suppression. Addition of either zwittermicin A or antibiotic B to alfalfa plants inoculated with a culture of a nonsuppressive mutant resulted in disease suppression. These results demonstrate that B. cereus UW85 produces two fungistatic antibiotics that contribute to suppression of damping-off disease of alfalfa.
Full text
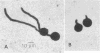
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Battisti L., Green B. D., Thorne C. B. Mating system for transfer of plasmids among Bacillus anthracis, Bacillus cereus, and Bacillus thuringiensis. J Bacteriol. 1985 May;162(2):543–550. doi: 10.1128/jb.162.2.543-550.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dion H. W., Woo P. W., Willmer N. E., Kern D. L., Onaga J., Fusari S. A. Butirosin, a new aminoglycosidic antibiotic complex: isolation and characterization. Antimicrob Agents Chemother. 1972 Aug;2(2):84–88. doi: 10.1128/aac.2.2.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halverson L. J., Handelsman J. Enhancement of soybean nodulation by Bacillus cereus UW85 in the field and in a growth chamber. Appl Environ Microbiol. 1991 Sep;57(9):2767–2770. doi: 10.1128/aem.57.9.2767-2770.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handelsman J., Raffel S., Mester E. H., Wunderlich L., Grau C. R. Biological Control of Damping-Off of Alfalfa Seedlings with Bacillus cereus UW85. Appl Environ Microbiol. 1990 Mar;56(3):713–718. doi: 10.1128/aem.56.3.713-718.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horii S., Nogami I., Mizokami N., Arai Y., Yoneda M. New antibiotic produced by bacteria, 5-beta-D-xylofuranosylneamine. Antimicrob Agents Chemother. 1974 Jun;5(6):578–581. doi: 10.1128/aac.5.6.578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peypoux F., Besson F., Michel G., Delcambe L. Structure of bacillomycin D, a new antibiotic of the iturin group. Eur J Biochem. 1981 Aug;118(2):323–327. doi: 10.1111/j.1432-1033.1981.tb06405.x. [DOI] [PubMed] [Google Scholar]
- Powell S. J., Prosser J. I. Protection of Nitrosomonas europaea colonizing clay minerals from inhibition by nitrapyrin. J Gen Microbiol. 1991 Aug;137(8):1923–1929. doi: 10.1099/00221287-137-8-1923. [DOI] [PubMed] [Google Scholar]
- Sengupta S., Banerjee A. B., Bose S. K. Gamma-glutamyl and D- or L-peptide linkages in mycobacillin, a cyclic peptide antibiotic. Biochem J. 1971 Mar;121(5):839–846. doi: 10.1042/bj1210839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TREVELYAN W. E., PROCTER D. P., HARRISON J. S. Detection of sugars on paper chromatograms. Nature. 1950 Sep 9;166(4219):444–445. doi: 10.1038/166444b0. [DOI] [PubMed] [Google Scholar]
- Tate M. E. Determination of ionization constants by paper electrophoresis. Biochem J. 1981 May 1;195(2):419–426. doi: 10.1042/bj1950419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomashow L. S., Weller D. M. Role of a phenazine antibiotic from Pseudomonas fluorescens in biological control of Gaeumannomyces graminis var. tritici. J Bacteriol. 1988 Aug;170(8):3499–3508. doi: 10.1128/jb.170.8.3499-3508.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanneste J. L., Yu J., Beer S. V. Role of antibiotic production by Erwinia herbicola Eh252 in biological control of Erwinia amylovora. J Bacteriol. 1992 May;174(9):2785–2796. doi: 10.1128/jb.174.9.2785-2796.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voisard C., Keel C., Haas D., Dèfago G. Cyanide production by Pseudomonas fluorescens helps suppress black root rot of tobacco under gnotobiotic conditions. EMBO J. 1989 Feb;8(2):351–358. doi: 10.1002/j.1460-2075.1989.tb03384.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youngman P. J., Perkins J. B., Losick R. Genetic transposition and insertional mutagenesis in Bacillus subtilis with Streptococcus faecalis transposon Tn917. Proc Natl Acad Sci U S A. 1983 Apr;80(8):2305–2309. doi: 10.1073/pnas.80.8.2305. [DOI] [PMC free article] [PubMed] [Google Scholar]