Abstract
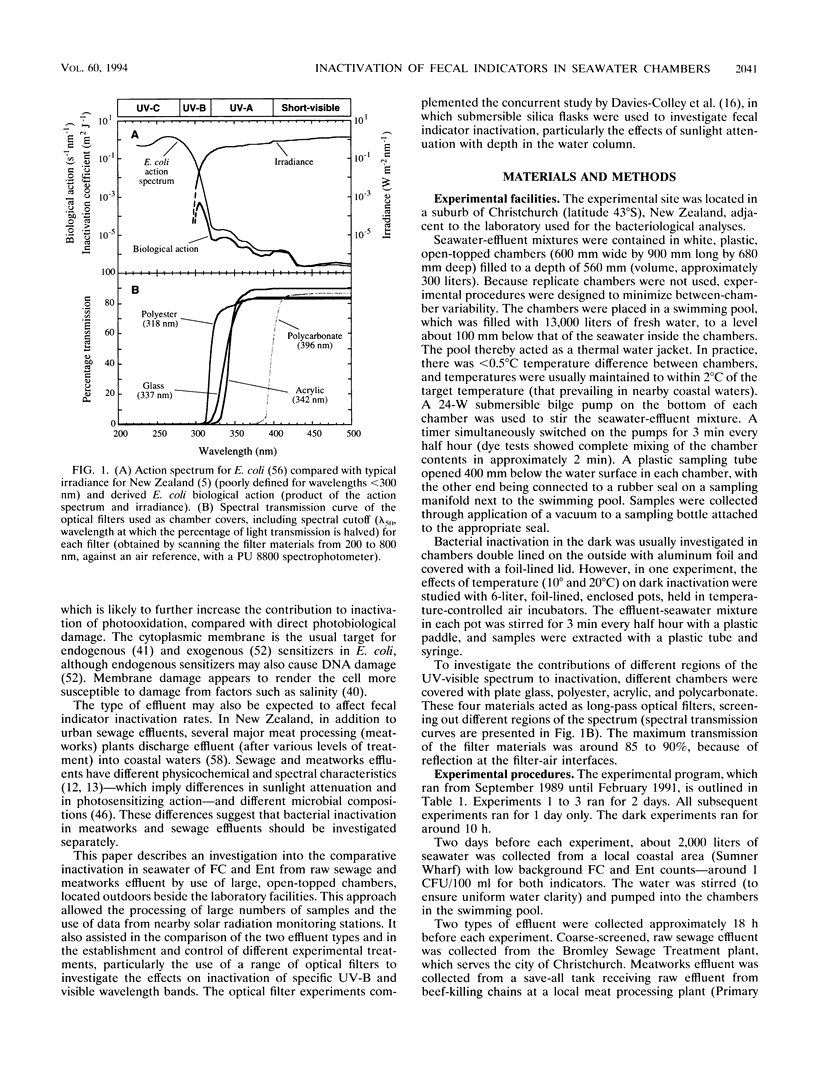
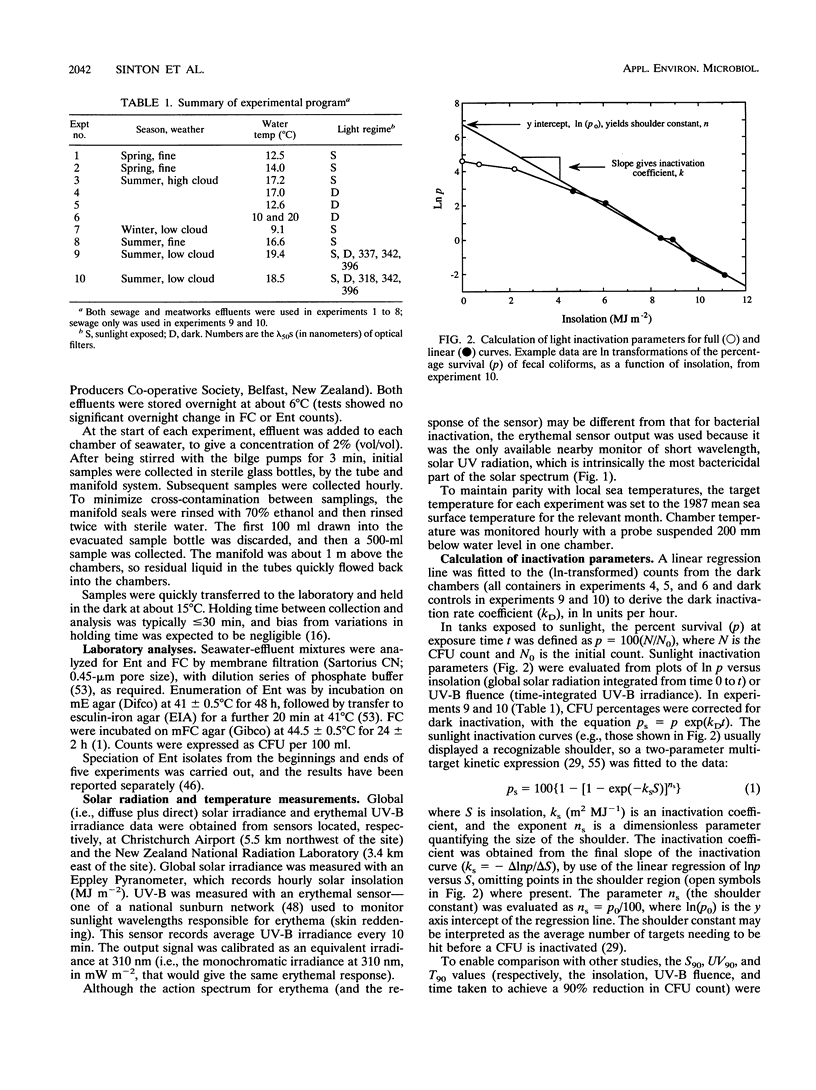
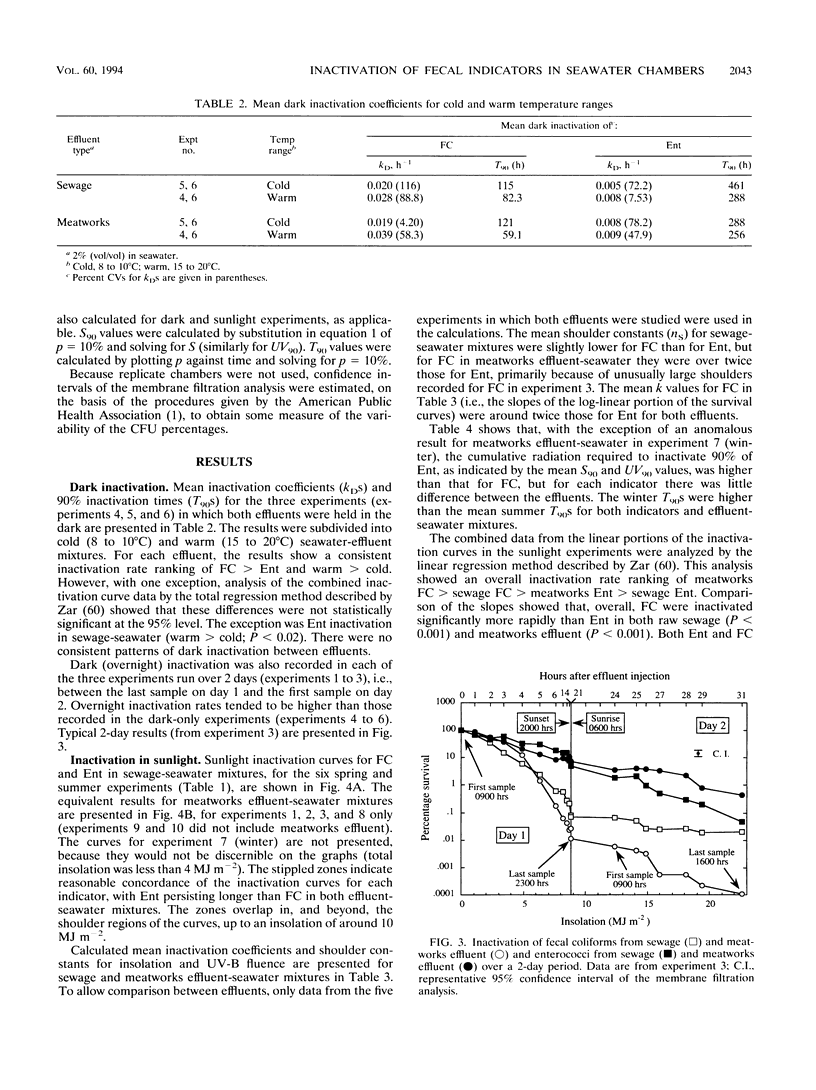
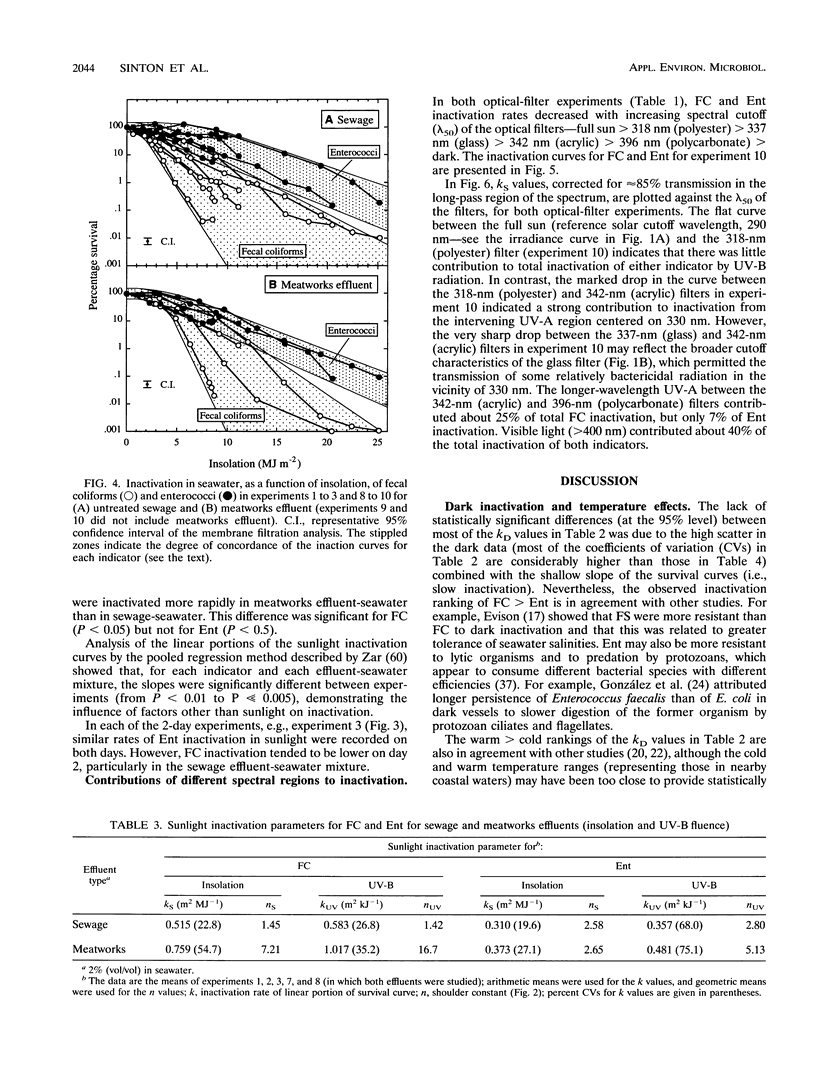
Inactivation in sunlight of fecal coliforms (FC) and enterococci (Ent) from sewage and meatworks effluents was measured in 300-liter effluent-seawater mixtures (2% vol/vol) held in open-topped chambers. Dark inactivation rates (kDs) were measured (from log-linear survival curves) in enclosed chambers and 6-liter pots. The kD for FC was 2 to 4 times that for Ent, and inactivation was generally slower at lower temperatures. Sunlight inactivation was described in terms of shoulder size (n) and the slope (k) of the log-linear portion of the survival curve as a function of global solar insolation and UV-B fluence. The n values tended to be larger for Ent than for FC, and the k values for FC were around twice those for Ent in both effluent-seawater mixtures. The combined sunlight data showed a general inactivation rate (k) ranking in effluent-seawater mixtures of meatworks FC > sewage FC > meatworks Ent > sewage Ent. Describing 90% inactivation in terms of insolation (S90) gave far less seasonal variation than T90 (time-dependent) values. However, there were significant differences in inactivation rates between experiments, indicating the contribution to inactivation of factors other than insolation. Inactivation rates under different long-pass optical filters decreased with the increase in the spectral cutoff wavelength (lambda 50) of the filters and indicated little contribution by UV-B to total inactivation. Most inactivation appeared to be caused by two main regions of the solar spectrum--between 318 and 340 nm in the UV region and > 400 nm in the visible region.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson I. C., Rhodes M. W., Kator H. I. Seasonal variation in survival of Escherichia coli exposed in situ in membrane diffusion chambers containing filtered and nonfiltered estuarine water. Appl Environ Microbiol. 1983 Jun;45(6):1877–1883. doi: 10.1128/aem.45.6.1877-1883.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson I. C., Rhodes M., Kator H. Sublethal stress in Escherichia coli: a function of salinity. Appl Environ Microbiol. 1979 Dec;38(6):1147–1152. doi: 10.1128/aem.38.6.1147-1152.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis T. P., Mara D. D., Silva S. A. Influence of pH, Oxygen, and Humic Substances on Ability of Sunlight To Damage Fecal Coliforms in Waste Stabilization Pond Water. Appl Environ Microbiol. 1992 Apr;58(4):1335–1343. doi: 10.1128/aem.58.4.1335-1343.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies-Colley R. J., Bell R. G., Donnison A. M. Sunlight inactivation of enterococci and fecal coliforms in sewage effluent diluted in seawater. Appl Environ Microbiol. 1994 Jun;60(6):2049–2058. doi: 10.1128/aem.60.6.2049-2058.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies C. M., Evison L. M. Sunlight and the survival of enteric bacteria in natural waters. J Appl Bacteriol. 1991 Mar;70(3):265–274. doi: 10.1111/j.1365-2672.1991.tb02935.x. [DOI] [PubMed] [Google Scholar]
- Fujioka R. S., Narikawa O. T. Effect of sunlight on enumeration of indicator bacteria under field conditions. Appl Environ Microbiol. 1982 Aug;44(2):395–401. doi: 10.1128/aem.44.2.395-401.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González J. M., Iriberri J., Egea L., Barcina I. Differential rates of digestion of bacteria by freshwater and marine phagotrophic protozoa. Appl Environ Microbiol. 1990 Jun;56(6):1851–1857. doi: 10.1128/aem.56.6.1851-1857.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimes D. J., Atwell R. W., Brayton P. R., Palmer L. M., Rollins D. M., Roszak D. B., Singleton F. L., Tamplin M. L., Colwell R. R. The fate of enteric pathogenic bacteria in estuarine and marine environments. Microbiol Sci. 1986 Nov;3(11):324–329. [PubMed] [Google Scholar]
- Hanes N. B., Fragala R. Effect of seawater concentration on survival of indicator bacteria. J Water Pollut Control Fed. 1967 Jan;39(1):97–104. [PubMed] [Google Scholar]
- Kelland L. R., Moss S. H., Davies D. J. Leakage of 86Rb+ after ultraviolet irradiation of Escherichia coli K-12. Photochem Photobiol. 1984 Mar;39(3):329–335. doi: 10.1111/j.1751-1097.1984.tb08186.x. [DOI] [PubMed] [Google Scholar]
- McCambridge J., McMeekin T. A. Effect of solar radiation and predacious microorganisms on survival of fecal and other bacteria. Appl Environ Microbiol. 1981 May;41(5):1083–1087. doi: 10.1128/aem.41.5.1083-1087.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss S. H., Smith K. C. Membrane damage can be a significant factor in the inactivation of Escherichia coli by near-ultraviolet radiation. Photochem Photobiol. 1981 Feb;33(2):203–210. doi: 10.1111/j.1751-1097.1981.tb05325.x. [DOI] [PubMed] [Google Scholar]
- Peak J. G., Peak M. J., Tuveson R. W. Ultraviolet action spectra for aerobic and anaerobic inactivation of Escherichia coli strains specifically sensitive and resistant to near ultraviolet radiations. Photochem Photobiol. 1983 Nov;38(5):541–543. doi: 10.1111/j.1751-1097.1983.tb03380.x. [DOI] [PubMed] [Google Scholar]
- Rhodes M. W., Kator H. Survival of Escherichia coli and Salmonella spp. in estuarine environments. Appl Environ Microbiol. 1988 Dec;54(12):2902–2907. doi: 10.1128/aem.54.12.2902-2907.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SLANETZ L. W., BARTLEY C. H. SURVIVAL OF FECAL STREPTOCCOCCI IN SEA WATER. Health Lab Sci. 1965 Jul;2:142–148. [PubMed] [Google Scholar]
- Smith G. J., White M. G., Ryan K. G. Seasonal trends in erythemal and carcinogenic ultraviolet radiation at mid-southern latitudes 1989-1991. Photochem Photobiol. 1993 Mar;57(3):513–517. doi: 10.1111/j.1751-1097.1993.tb02328.x. [DOI] [PubMed] [Google Scholar]
- Sprott G. D., Martin W. G., Schneider H. Differential effects of near-UV and visible light on active transport and other membrane processes in Escherichia coli. Photochem Photobiol. 1976 Jul;24(1):21–27. doi: 10.1111/j.1751-1097.1976.tb06793.x. [DOI] [PubMed] [Google Scholar]
- Tuveson R. W., Larson R. A., Kagan J. Role of cloned carotenoid genes expressed in Escherichia coli in protecting against inactivation by near-UV light and specific phototoxic molecules. J Bacteriol. 1988 Oct;170(10):4675–4680. doi: 10.1128/jb.170.10.4675-4680.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasconcelos G. J., Swartz R. G. Survival of bacteria in seawater using a diffusion chamber apparatus in situ. Appl Environ Microbiol. 1976 Jun;31(6):913–920. doi: 10.1128/aem.31.6.913-920.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb R. B., Brown M. S. Action spectra for oxygen-dependent and independent inactivation of Escherichia coli WP2s from 254 to 460 nm. Photochem Photobiol. 1979 Feb;29(2):407–409. doi: 10.1111/j.1751-1097.1979.tb07068.x. [DOI] [PubMed] [Google Scholar]
- Webb R. B., Brown M. S. Sensitivity of strains of Escherichia coli differing in repair capability to far UV, near UV and visible radiations. Photochem Photobiol. 1976 Nov;24(5):425–432. doi: 10.1111/j.1751-1097.1976.tb06849.x. [DOI] [PubMed] [Google Scholar]



