Abstract
Oxytocin induces mating behavior in rats of both sexes. Previous experiments revealed that progesterone-induced sex behavior in ovariectomized, estrogen-primed rats was caused by release of NO from NOergic neurons that stimulated the release of luteinizing hormone-releasing hormone (LHRH). The LHRH activated brain-stem neurons that initiated the lordosis reflex. We hypothesized that oxytocin might similarly release NO in the medial basal hypothalamic region that would stimulate release of LHRH into the hypophyseal portal vessels to release luteinizing hormone. To investigate this hypothesis, medial basal hypothalamic explants were preincubated in Krebs–Ringer bicarbonate buffer for 30 min, followed by a 30-min incubation in fresh Krebs–Ringer bicarbonate buffer containing the compounds to be tested. Oxytocin stimulated LHRH release 3- to 4-fold at the lowest concentration tested (10−10 M). Values remained at a plateau as the concentration was increased to 10−7 M and then declined in a concentration-dependent manner, so that there was no stimulation with a concentration of 10−5 M. Oxytocin (10−7 M) stimulated release of prostaglandin E2 into the medium, a finding consistent with a role of NO in the response. That NO indeed mediated the action of oxytocin was supported by blockade of the action of oxytocin by the competitive inhibitor of NO synthase (NOS), NG-monomethyl-l-arginine (300 μM). Furthermore, oxytocin (10−9 to 10−7 M) activated NOS as measured at the end of the experiments. Oxytocin appeared to act to stimulate norepinephrine terminals in the medial basal hypothalamus, which activated NOS by α1-adrenergic receptors, because prazocine, an α1 receptor blocker, inhibited the LHRH-releasing action of oxytocin. Finally, incubation of neural lobe explants with sodium nitroprusside, a NO releasor, revealed that nitroprusside (300–600 μM, but not 900 μM) inhibited oxytocin release. Therefore, the NO released by oxytocin also diffuses into the oxytocin neuronal endings and inhibits oxytocin release, forming a negative feedback loop. The results indicate that oxytocin is important not only in induction of mating, but also in stimulating LHRH release with subsequent luteinizing hormone discharge that plays a crucial role in reproduction.
Although oxytocin is one of the most abundant peptides in the body, little is known about its function. In the female, it is critical for milk ejection during lactation and plays an important role in parturition by inducing uterine contractions (1, 2). Oxytocin also appears to influence maternal and mating behavior, because intraventricular injection of the peptide induces these behaviors in female rats (3, 4).
Although the amounts of oxytocin present in the neurohypophysis of male and female rats are similar, its function in the male has not been established (1). It induces natriuresis in both sexes (1), and recent evidence indicates that it may do so by releasing atrial natriuretic peptide (5).
Intraventricular injection of oxytocin causes penile erection in male rats. This effect can be inhibited by injecting inhibitors of nitric oxide synthase (NOS) into the cerebral ventricles (6). Mating behavior in female rats, castrated and treated with estrogen, can be evoked by progesterone, and this effect can be blocked by inhibition of NOS (7). Because this behavior is induced by the release of luteinizing hormone-releasing hormone (LHRH) into brain-stem regions that provoke lordosis behavior, it appears that NO releases the LHRH required for mating behavior (7). NO is known to release LHRH into the hypophyseal portal vessels, thus inducing the release of luteinizing hormone from the pituitary gland (8, 9).
Because of these associations, we hypothesized that oxytocin controls release of LHRH into the portal vessels, thereby stimulating release of luteinizing hormone from the anterior pituitary gland of rats of both sexes (8). Furthermore, because LHRH release is controlled by NO, it also might mediate the LHRH-releasing action of oxytocin. Therefore, studies were undertaken to determine the possible influence of oxytocin in release of LHRH in the male and whether NO is important to the process.
NOS-containing neurons (termed NOergic neurons) are closely related both to the oxytocinergic and LHRHergic neuronal systems. In the case of the former, many of the oxytocin neurons in the paraventricular and supraoptic nucleus also contain NOS (10), and the enzyme not only is produced in the cell bodies of these neurons, but also is present in abundance in the axon terminals in the neural lobe, which have a higher concentration of NOS than in any other organ in the rat (10). Additionally, there is a plexus of NOergic fibers and cell bodies in the region of the arcuate nucleus in juxtaposition to the axons of the LHRH neurons (9). It is established that NO controls the release of LHRH (8). Therefore, the anatomical substrate for the postulated interactions is present in the medial basal hypothalamus (MBH). The role and mechanism of action of oxytocin in the release of LHRH from incubated MBH explants were studied in the present experiments.
MATERIALS AND METHODS
Male rats of the Wistar strain (200–250 g) from our colony were used. All rats were kept two to a cage in a light (0500–1900)- and temperature (23–25°C)-controlled room with free access to laboratory chow and tap water.
After decapitation and removal of the brain, the MBH was dissected by making a transverse cut just behind the optic chasm, extending dorsally 1.0 mm. A horizontal cut extended from this point caudally to just behind the pituitary stalk, where another frontal cut was made. Bilateral longitudinal cuts were made 1 mm lateral to the midline. All procedures were approved by the Institutional Animal Research Review Committee.
The hypothalami were preincubated in Krebs–Ringer bicarbonate-buffered medium (pH 7.4) containing 0.1% glucose for 30 min before replacement with fresh medium or medium containing the substances to be tested. The incubation was continued for 30 min followed by removal of the medium, heating in a boiling water bath for 10 min, and storage of samples at −20°C before assay for LHRH. Incubations were carried out in a Dubnoff metabolic shaker (50 cycles per min; 95% O2/5% CO2) (9). LHRH was measured, as in previous studies (9), by RIA using a highly specific LHRH antiserum kindly provided by A. Barnea (University of Texas Southwestern Medical Center, Dallas). The sensitivity of the assay was 0.2 pg per tube, and the curve was linear up to 100 pg of LHRH.
Prostaglandin E2 (PGE2) also was measured in media in certain experiments by RIA (Peninsula Laboratories).
For determination of NO release from the incubated MBH explants, we used a modification of the method of Bredt and Snyder (11). At the end of the incubation, the explant is homogenized and [14C]arginine is added and incubated for 15 min under optimal conditions at 37°C. The conversion of [14C]arginine to [14C]citrulline is measured. Because citrulline remains in the sample, whereas the equimolar amounts of NO produced are rapidly destroyed, this provides a measure of the activity of the enzyme. We have shown that this modification provides an estimate of the quantity of the enzyme in the sample (9).
For incubation of the neural lobe of the pituitary gland, each was removed from the pituitary and placed in an individual tube containing 500 μl of Krebs–Ringer bicarbonate buffer (KRB). The neural lobes were preincubated for 30 min and then incubated for another 30 min in fresh KRB containing the compounds to be tested. The oxytocin released into the medium was measured by RIA using a kit supplied by Peninsula Laboratories. All experiments were repeated at least once.
Sodium nitroprusside (NP), NG-monomethyl-l-arginine (NMMA), and hemoglobin were purchased from Sigma. Oxytocin was obtained from Peninsula Laboratories.
Statistics.
Data were analyzed using one-way analysis of variance techniques and the Student–Newman–Keuls test for multiple comparisons. Differences with P values < 0.05 were considered significant.
RESULTS
Effect of Different Concentrations of Oxytocin on LHRH Release from MBH Explants.
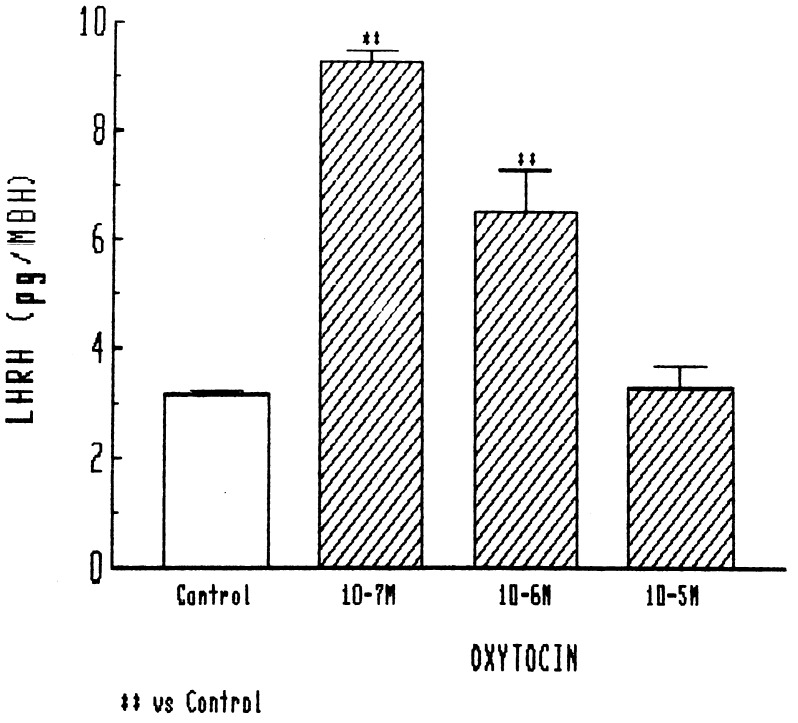
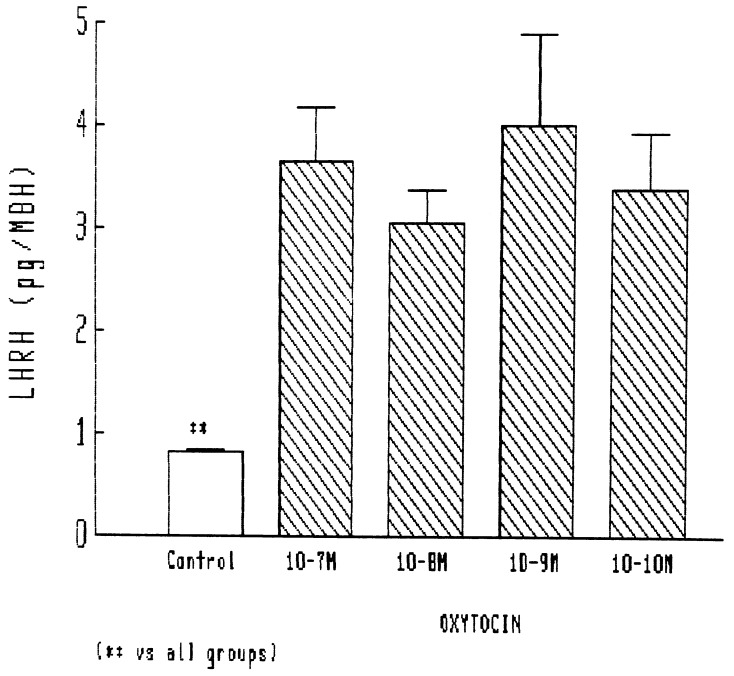
In the initial experiments, relatively high concentrations of oxytocin were tested, and the lowest concentration used (10−7 M) caused a highly significant 3-fold increase in LHRH release. Release was less, but still significant with 10−6 M and at basal levels with 10−5 M (Fig. 1). Therefore, in other experiments, we tested doses that were much lower than those in the initial experiments. In these experiments, a highly significant release, increasing basal release by nearly 4-fold, was obtained with 10−7 M. As the concentration was decreased to 10−10 M, there was no reduction in the highly significant response (Fig. 2). Thus, the MBH is extremely sensitive to the LHRH-releasing action of oxytocin in the male rat, but relatively high concentrations result in loss of stimulation.
Figure 1.
Effect of different concentrations of oxytocin on LHRH release from MBH explants. In this and subsequent figures, the height of the column indicates the mean, and the vertical line and horizontal bar represent 1 SEM. There were 6 or more determinations per group. ∗∗, P < 0.001 vs. control.
Figure 2.
Stimulatory effect of lower concentrations of oxytocin on LHRH release from MBH. ∗∗, P < 0.001 vs. all other groups.
Effect of Oxytocin on PGE2 Release from MBH Explants.
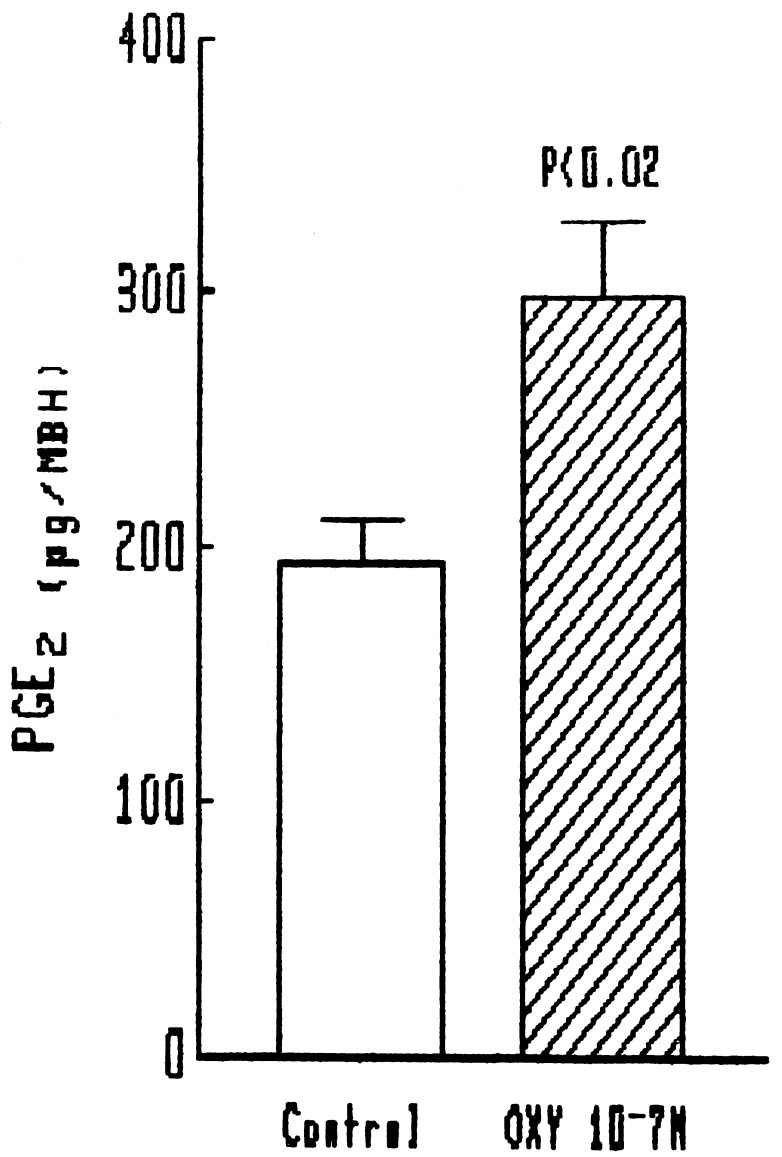
Because the stimulatory effect of NO on LHRH release is mediated by activation of cyclooxygenase and production of PGE2 (8, 9), we determined if oxytocin increases PGE2 release into the medium of the explants. A significant release occurred with an oxytocin concentration of 10−7 M (Fig. 3).
Figure 3.
Effect of oxytocin on PGE2 release from MBH.
Effect of Inhibition of NOS by NMMA on LHRH Release from MBH Explants.
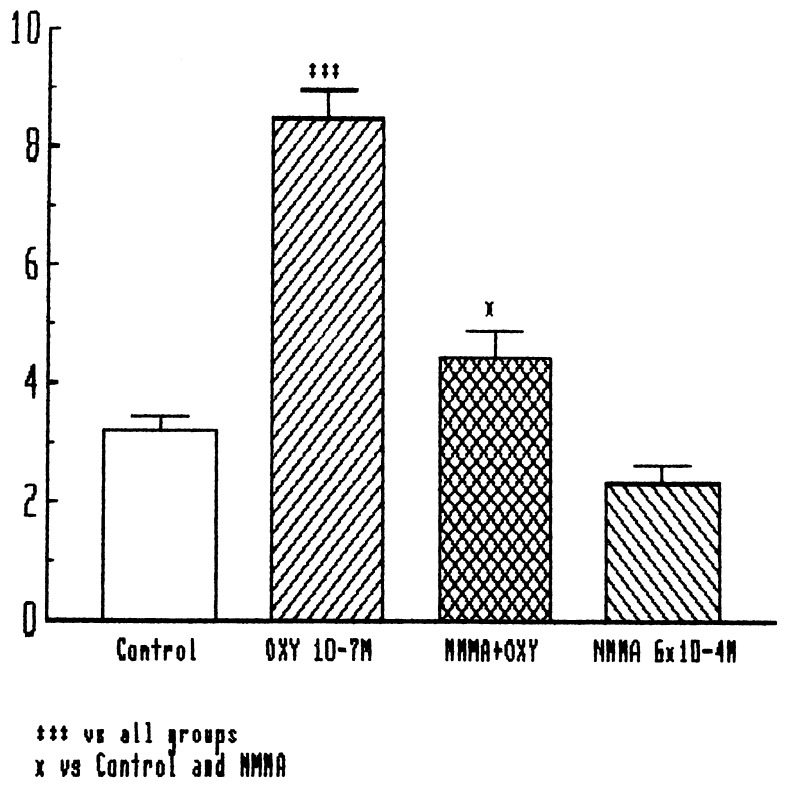
NMMA, an analog of arginine, is a competitive inhibitor of NOS that blocks its conversion of arginine into citrulline and NO. At the concentration used in other studies (300 μM) (8, 9), it had no significant effect on basal release of LHRH, but almost completely blocked the release of LHRH induced by oxytocin (Fig. 4).
Figure 4.
Effect of oxytocin and NMMA (300 μM) on LHRH release from MBH. ∗∗∗, P < 0.001 vs. all other groups. ∗, P < 0.05 vs. control and NMMA groups.
Effect of Oxytocin on NOS Activity in MBH Explants.
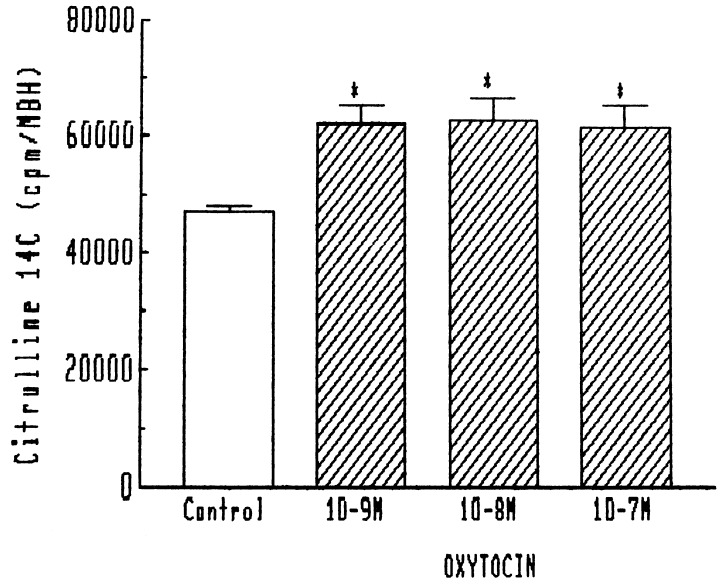
Other stimuli, which increased LHRH release through NO, such as norepinephrine and N-methyl-d-aspartic acid, also increased the activity of NOS as measured by the modified citrulline method at the end of the experiment. Consequently, we tested the effect of oxytocin on NOS activity at the termination of the experiments. Indeed, it increased the activity of NOS in the same dose range, which increased the release of LHRH (10−7 to 10−9 M) (Fig. 5).
Figure 5.
Effect of oxytocin on NOS activity in MBH. ∗, P < 0.01 vs. control.
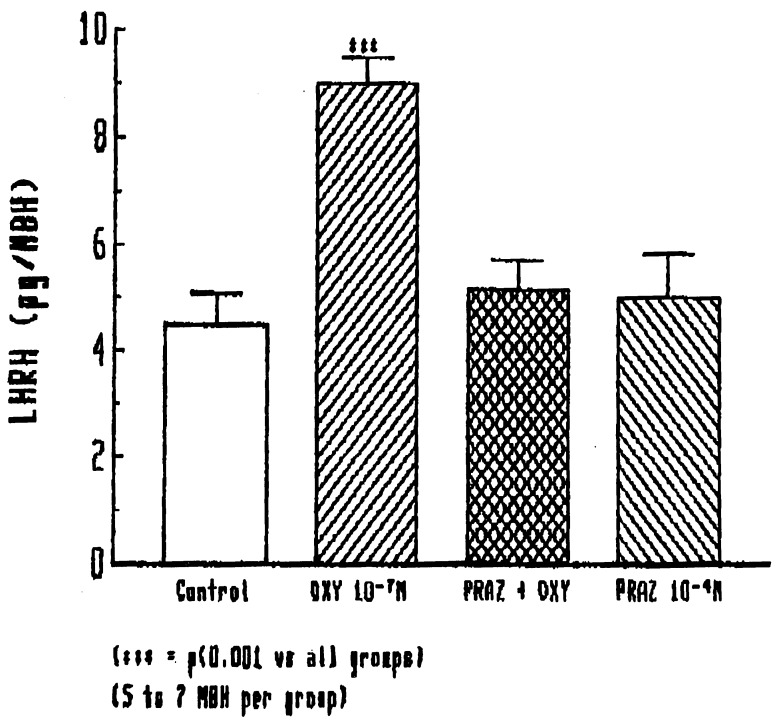
Effect of Prazocin on Oxytocin-Induced LHRH Release.
Because our earlier experiments (8) indicated that NO-induced LHRH release was mediated by norepinephrine, we hypothesized that oxytocin might release LHRH by induction of norepinephrine release. Consequently, we determined the effect of prazosin, an α1-adrenergic receptor blocker, on the LHRH release induced by oxytocin. Indeed, prazocin, which previous experiments had shown blocked the norepinephrine-induced but not basal LHRH release, had no effect on basal release but completely blocked oxytocin (10−7 M)-induced LHRH release (Fig. 6).
Figure 6.
Effect of prazocin (10−4 M) on oxytocin-induced stimulation of LHRH release from the MBH.
Effect of NO on Oxytocin Release from the Neural Lobe.
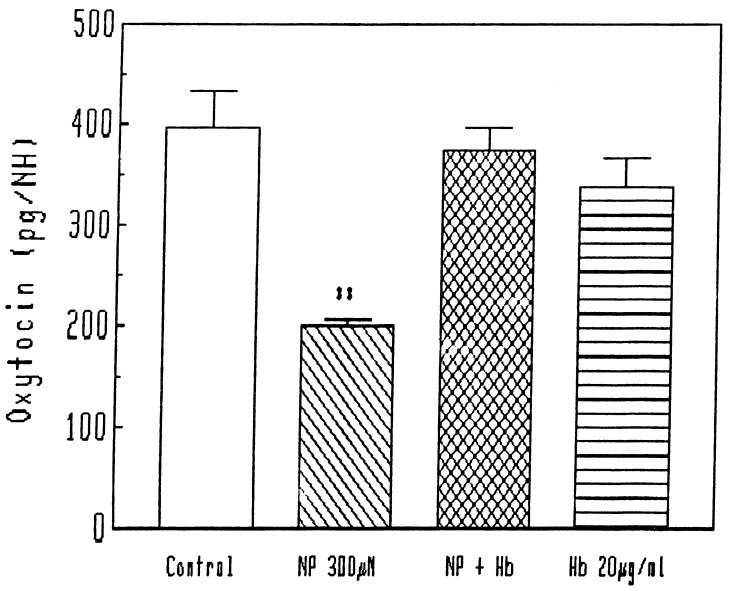
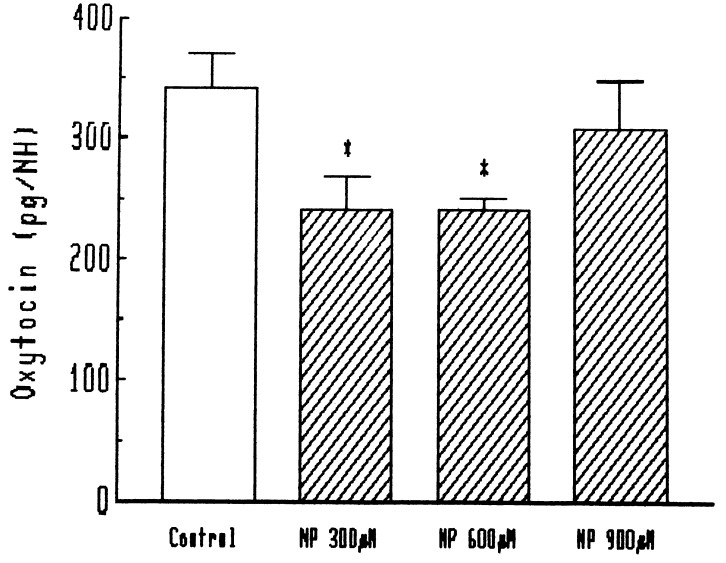
We recently discovered a number of feedbacks in the NOergic system controlling LHRH, and consequently we evaluated the effect of NO released by NP on the release of oxytocin from the oxytocinergic terminals in the neurohypophysis. NP spontaneously releases NO, and, at the concentrations tested here (300 and 600 μM), concentrations that are highly effective in release of LHRH (11), NO induced a highly significant reduction in the release of oxytocin from the neural lobe (Fig. 7, 8). When a scavenger of NO, hemoglobin, was added to the preparation, there was no effect on the basal release, but the effect of NP was completely blocked (Fig. 7). When various concentrations of NP were tested, the inhibitory effect on oxytocin release vanished at a concentration of 900 μM (Fig. 8).
Figure 7.
Effect of NP and hemoglobin (Hb) on oxytocin release from neurohypophysis (NH). ∗∗, P < 0.001 vs. all other groups.
Figure 8.
Effect of different concentrations of NP on oxytocin release from neurohypophysis (NH). ∗, P < 0.01 vs. control.
DISCUSSION
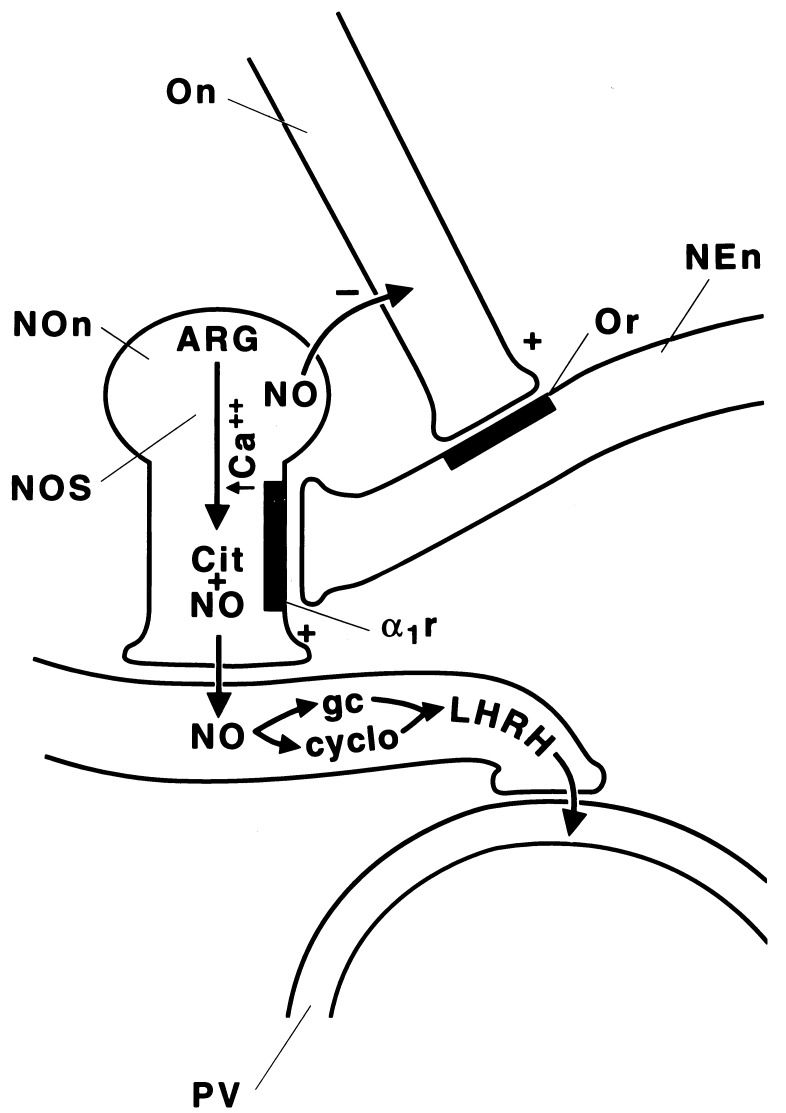
These results show that oxytocin can release LHRH from MBH explants of male rats, and that it is active at very low concentrations that certainly could exist in vivo. Furthermore, its action is similar to that of other stimulants of LHRH release, because it is accompanied by PGE2 release. The action appears to result from release of NO, because blockade of NOS with NMMA blocked the stimulant action of oxytocin. In addition, oxytocin increased the content of the enzyme measured by the citrulline method. Presumably, as in the case of norepinephrine and N-methyl-d-aspartic acid (8), oxytocin stimulates, either directly or indirectly, the NOergic neurons, which are activated by an increase in intracellular free calcium, which combines with calmodulin and activates neural NOS (9) (Fig. 9). The enzyme causes conversion of arginine into citrulline plus NO. The NO diffuses to the LHRH terminal and stimulates it by its combined activation of guanylate cyclase and also cyclooxygenase as described earlier (9). The increase in cGMP presumably increases intracellular free calcium concentrations to activate phospholipaseA2, which converts membrane phospholipids into arachidonate that serves as substrate for the activated cyclooxygenase leading to the production of PGE2. PGE2 then activates adenylate cyclase leading to production of cAMP that activates protein kinase A, leading to extrusion of LHRH secretory granules.
Figure 9.
Schematic diagram of the mechanism of stimulation of LHRH release by oxytocin. NEn, noradrenergic neuron; α1r, α1-adrenergic receptor; Or, oxytocin receptor; On, oxytocin neuron; NOn, NOergic neuron; ARG, arginine; cit, citrulline; gc, guanylate cyclase; cyclo, cyclooxygenase; PV, portal vein.
The mechanism by which oxytocin activates the NOergic neuron is indirect: its stimulation of the release of norepinephrine acts by α1 receptors to activate NOS. Indeed, it has been shown that oxytocin releases norepinephrine in the hypothalamus (12) (Fig. 9). Determination of the physiologic significance of these effects will require use of oxytocin antagonists. However, the low concentrations, which were effective, suggest that the action is physiologically significant.
Interestingly, we also found that NO released from NP suppressed the release of oxytocin from the neural lobe, suggesting that there is a negative feedback mechanism by which oxytocin stimulates the release of NO, which in turn, acts back to inhibit oxytocin release. Indeed, Summy-Long et al. (13) reported that inhibition of NOS increased oxytocin release, in agreement with our findings. We recently reported that NO also suppresses dopamine and norepinephrine release from MBH explants and postulated that this could be a negative feedback mechanism to terminate pulsatile LHRH release driven by norepinephrine (14). The failure of high concentrations of oxytocin to release LHRH may be accounted for by the high concentrations of NO released, because a similar bell-shaped dose–response curve of LHRH release was obtained with increasing concentrations of NP (15).
Not only does oxytocin stimulate release of LHRH, which is bound for the pituitary gland where it releases luteinizing hormone, but also the LHRH release stimulated by oxytocin may play a role in mating behavior in the male rat, because intraventricular injection of oxytocin produced penile erection. This effect of oxytocin is induced by NO, because the response was blocked by intraventricular injection of NOS inhibitors (6). Thus, oxytocin has a major role in control of reproduction in the male rat that may be added to its probable role in the control of body fluid homeostasis (5).
Acknowledgments
We thank Judy Scott and Jason P. Holland for their excellent secretarial assistance. This work was supported by National Institutes of Health Grants DK43900 and MH51853.
ABBREVIATIONS
- LHRH
luteinizing hormone-releasing hormone
- NOS
nitric oxide synthase
- MBH
medial basal hypothalamus
- NP
sodium nitroprusside
- NMMA
NG-monomethyl-l-arginine
- PGE2
prostaglandin E2
References
- 1.Crowley W R, Armstrong W E. Endocr Rev. 1992;13:33–65. doi: 10.1210/edrv-13-1-33. [DOI] [PubMed] [Google Scholar]
- 2.Ivell R, Russell J A. Rev Reprod. 1996;1:13–18. doi: 10.1530/ror.0.0010013. [DOI] [PubMed] [Google Scholar]
- 3.Argiolas, A. & Gessa, G. L. Neurosci. Biobehav. Rev. (1994) 15, 217–231. [DOI] [PubMed]
- 4.Schulze H G, Gorzalka B B. Neuropeptides. 1991;18:99–106. doi: 10.1016/0143-4179(91)90008-7. [DOI] [PubMed] [Google Scholar]
- 5.Haanwinckel M A, Elias L K, Favaretto A L V, Gutkowska J, McCann S M, Antunes-Rodrigues J. Proc Natl Acad Sci USA. 1995;92:7902–7906. doi: 10.1073/pnas.92.17.7902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Melis M R, Argiolas A. Brain Res Bull. 1993;32:71–74. doi: 10.1016/0361-9230(93)90321-2. [DOI] [PubMed] [Google Scholar]
- 7.Mani S K, Allen J M C, Rettori V, O’Malley B W, Clark J H, McCann S M. Proc Natl Acad Sci USA. 1994;91:6468–6472. doi: 10.1073/pnas.91.14.6468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McCann S M, Rettori V. Proc Soc Exp Biol Med. 1996;211:7–15. doi: 10.3181/00379727-211-43950b. [DOI] [PubMed] [Google Scholar]
- 9.Canteros G, Rettori V, Franchi A, Genaro A, Cebral E, Faletti A, Gimeno M, McCann S M. Proc Natl Acad Sci USA. 1995;92:3416–3420. doi: 10.1073/pnas.92.8.3416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bredt D S, Hwang P M, Snyder S H. Nature (London) 1990;347:768–770. doi: 10.1038/347768a0. [DOI] [PubMed] [Google Scholar]
- 11.Bredt D S, Snyder S H. Proc Natl Acad Sci USA. 1989;86:9030–9033. doi: 10.1073/pnas.86.22.9030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Etgen A M, Karkanias G B. Psychoneuroendocrinology. 1994;19:603–610. doi: 10.1016/0306-4530(94)90044-2. [DOI] [PubMed] [Google Scholar]
- 13.Summy-Long J Y, Bui V, Mantz S, Kochler E, Weisz J, Kadeknro M. Neurosci Lett. 1993;152:190–193. doi: 10.1016/0304-3940(93)90515-m. [DOI] [PubMed] [Google Scholar]
- 14.Seilicovich A, Lasaga M, Befumo M, Duvilanski B H, Del M, Dias C, Rettori V, McCann S M. Proc Natl Acad Sci USA. 1995;92:11299–11302. doi: 10.1073/pnas.92.24.11299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Canteros G, Rettori V, Genaro A, Suburo A, Gimeno M, McCann S M. Proc Natl Acad Sci USA. 1996;93:4246–4250. doi: 10.1073/pnas.93.9.4246. [DOI] [PMC free article] [PubMed] [Google Scholar]