Abstract
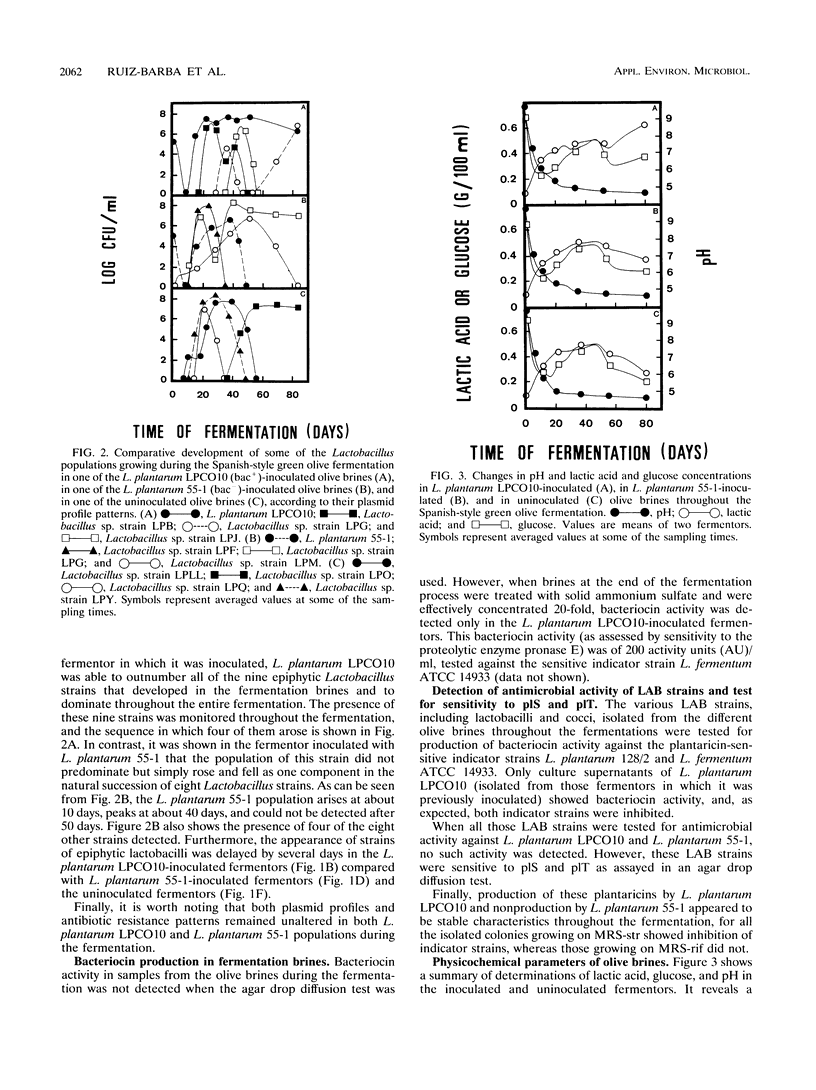
Bacteriocin-producing Lactobacillus plantarum LPCO10 and its non-bacteriocin-producing, bacteriocinimmune derivative, L. plantarum 55-1, were evaluated separately for growth and persistence in natural Spanish-style green olive fermentations. Both strains were genetically marked and selectively enumerated using antibiotic-containing media. Plasmid profile and bacteriocin production (bac+) were used as additional markers. When olive brines were inoculated at 105 CFU/ml, the parent strain, LPCO10, proliferated to dominate the epiphytic microflora, sharing high population levels with other spontaneously occurring lactobacilli and persisting throughout the fermentation (12 weeks). In contrast, the derivative strain could not be isolated after 7 weeks. Stability of both plasmid profile and bac+ (LPCO10 strain) or bac- (55-1 strain) phenotype was shown by L. plantarum LPCO10 and L. plantarum 55-1 isolated throughout the fermentation. Bacteriocin activity could be found in the L. plantarum LPCO10-inoculated brines only after ammonium sulfate precipitation and concentration (20 times) of the final brine. Spontaneously occurring lactobacilli and lactic coccus populations, which were isolated from each of the fermenting brines studied during this investigation, were shown to be sensitive to the bacteriocins produced by L. plantarum LPCO10 when tested by the drop diffusion test. The declines in both pH and glucose levels throughout the fermentative process were similar in L. plantarum LPCO10- and in L. plantarum 55-1-inoculated brines and were comparable to the declines in the uninoculated brines. However, the final concentration of lactic acid in L. plantarum LPCO10-inoculated brines was higher than in the L. plantarum 55-1-inoculated brines and uninoculated brines. These results indicated that L. plantarum LPCO10 may be useful as a starter culture to control the lactic acid fermentation of Spanish-style green olives.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson D. G., McKay L. L. Simple and rapid method for isolating large plasmid DNA from lactic streptococci. Appl Environ Microbiol. 1983 Sep;46(3):549–552. doi: 10.1128/aem.46.3.549-552.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balatsouras G., Tsibri A., Dalles T., Doutsias G. Effects of fermentation and its control on the sensory characteristics of conservolea variety green olives. Appl Environ Microbiol. 1983 Jul;46(1):68–74. doi: 10.1128/aem.46.1.68-74.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barefoot S. F., Klaenhammer T. R. Detection and activity of lactacin B, a bacteriocin produced by Lactobacillus acidophilus. Appl Environ Microbiol. 1983 Jun;45(6):1808–1815. doi: 10.1128/aem.45.6.1808-1815.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etchells J. L., Borg A. F., Kittel I. D., Bell T. A., Fleming H. P. Pure culture fermentation of green olives. Appl Microbiol. 1966 Nov;14(6):1027–1041. doi: 10.1128/am.14.6.1027-1041.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foegeding P. M., Thomas A. B., Pilkington D. H., Klaenhammer T. R. Enhanced control of Listeria monocytogenes by in situ-produced pediocin during dry fermented sausage production. Appl Environ Microbiol. 1992 Mar;58(3):884–890. doi: 10.1128/aem.58.3.884-890.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geis A., Singh J., Teuber M. Potential of lactic streptococci to produce bacteriocin. Appl Environ Microbiol. 1983 Jan;45(1):205–211. doi: 10.1128/aem.45.1.205-211.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris L. J., Fleming H. P., Klaenhammer T. R. Novel paired starter culture system for sauerkraut, consisting of a nisin-resistant Leuconostoc mesenteroides strain and a nisin-producing Lactococcus lactis strain. Appl Environ Microbiol. 1992 May;58(5):1484–1489. doi: 10.1128/aem.58.5.1484-1489.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiménez-Díaz R., Rios-Sánchez R. M., Desmazeaud M., Ruiz-Barba J. L., Piard J. C. Plantaricins S and T, Two New Bacteriocins Produced by Lactobacillus plantarum LPCO10 Isolated from a Green Olive Fermentation. Appl Environ Microbiol. 1993 May;59(5):1416–1424. doi: 10.1128/aem.59.5.1416-1424.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattila-Sandholm T., Haikara A., Skyttä E. The effect of Pediococcus damnosus and Pediococcus pentosaceus on the growth of pathogens in minced meat. Int J Food Microbiol. 1991 May;13(1):87–94. doi: 10.1016/0168-1605(91)90141-b. [DOI] [PubMed] [Google Scholar]
- Ruiz-Barba J. L., Piard J. C., Jiménez-Díaz R. Plasmid profiles and curing of plasmids in Lactobacillus plantarum strains isolated from green olive fermentations. J Appl Bacteriol. 1991 Nov;71(5):417–421. doi: 10.1111/j.1365-2672.1991.tb03810.x. [DOI] [PubMed] [Google Scholar]
- Schillinger U., Kaya M., Lücke F. K. Behaviour of Listeria monocytogenes in meat and its control by a bacteriocin-producing strain of Lactobacillus sake. J Appl Bacteriol. 1991 Jun;70(6):473–478. doi: 10.1111/j.1365-2672.1991.tb02743.x. [DOI] [PubMed] [Google Scholar]
- Sharp R., O'donnell A. G., Gilbert H. G., Hazlewood G. P. Growth and Survival of Genetically Manipulated Lactobacillus plantarum in Silage. Appl Environ Microbiol. 1992 Aug;58(8):2517–2522. doi: 10.1128/aem.58.8.2517-2522.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkowski K., Crandall A. D., Montville T. J. Inhibition of Listeria monocytogenes by Lactobacillus bavaricus MN in beef systems at refrigeration temperatures. Appl Environ Microbiol. 1993 Aug;59(8):2552–2557. doi: 10.1128/aem.59.8.2552-2557.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yousef A. E., Luchansky J. B., Degnan A. J., Doyle M. P. Behavior of Listeria monocytogenes in wiener exudates in the presence of Pediococcus acidilactici H or pediocin AcH during storage at 4 or 25 degrees C. Appl Environ Microbiol. 1991 May;57(5):1461–1467. doi: 10.1128/aem.57.5.1461-1467.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwietering M. H., Jongenburger I., Rombouts F. M., van 't Riet K. Modeling of the bacterial growth curve. Appl Environ Microbiol. 1990 Jun;56(6):1875–1881. doi: 10.1128/aem.56.6.1875-1881.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]



