Abstract
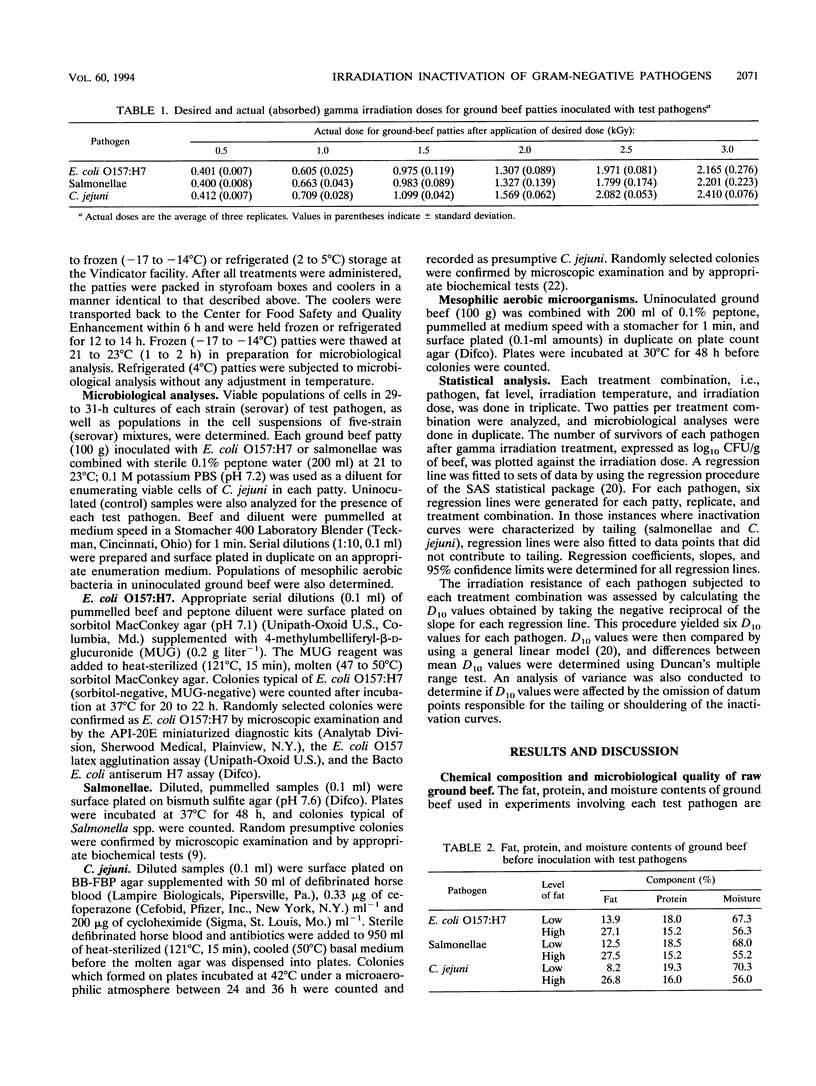
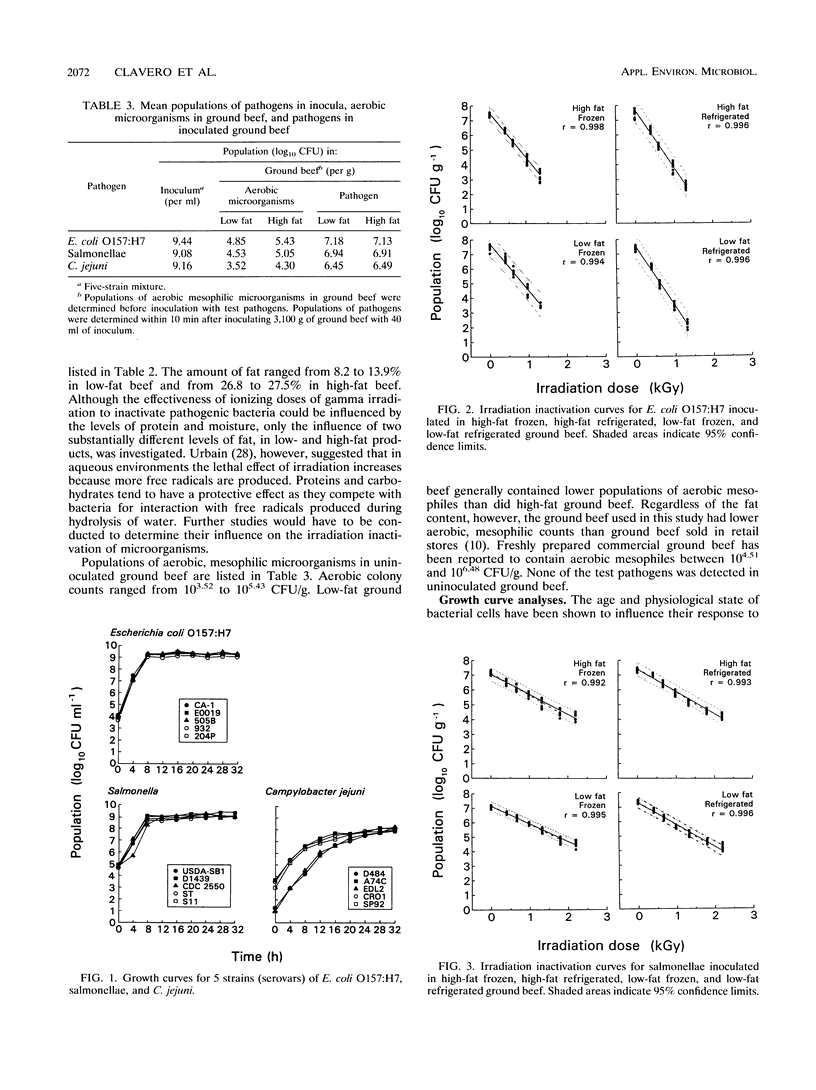
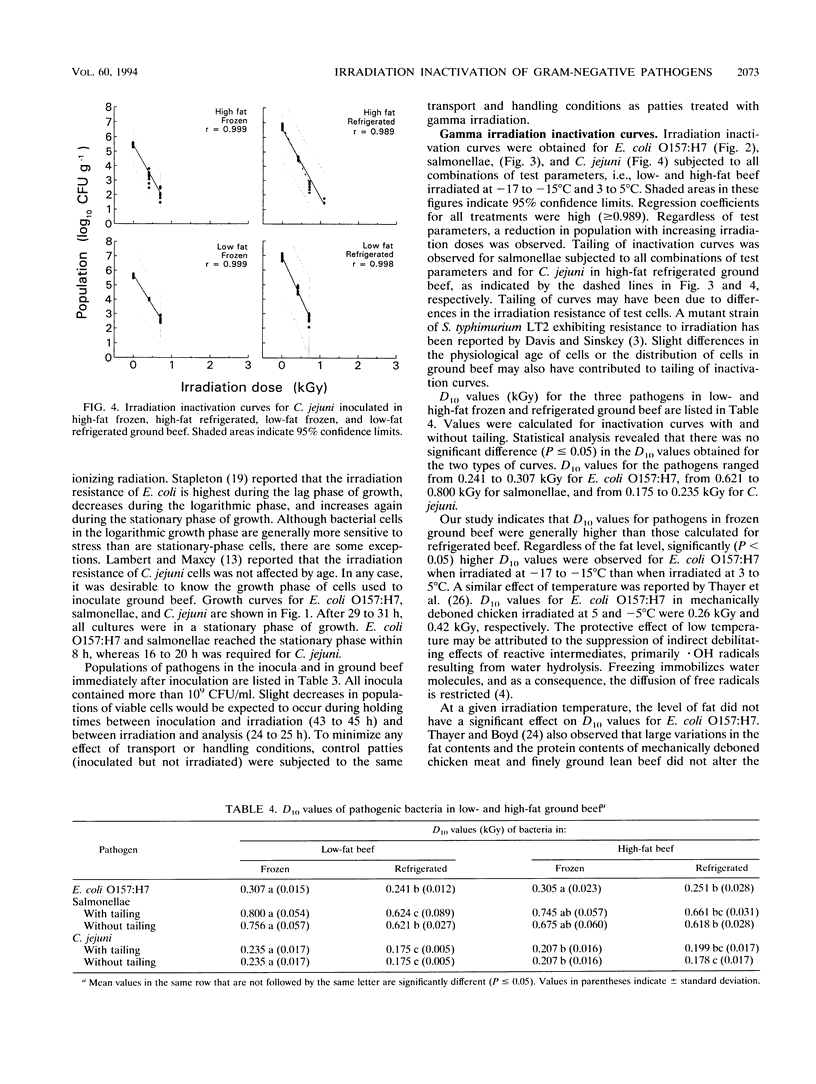
Raw ground beef patties inoculated with stationary-phase cells of Escherichia coli O157:H7, salmonellae, or Campylobacter jejuni were subjected to gamma irradiation (60Co) treatment, with doses ranging from 0 to 2.52 kGy. The influence of two levels of fat (8 to 14% [low fat] and 27 to 28% [high fat]) and temperature (frozen [-17 to -15 degrees C] and refrigerated [3 to 5 degrees C]) on the inactivation of each pathogen by irradiation was investigated. In ascending order of irradiation resistance, the D10 values ranged from 0.175 to 0.235 kGy (C. jejuni), from 0.241 to 0.307 kGy (E. coli O157:H7), and from 0.618 to 0.800 kGy (salmonellae). Statistical analysis revealed that E. coli O157:H7 had a significantly (P < 0.05) higher D10 value when irradiated at -17 to -15 degrees C than when irradiated at 3 to 5 degrees C. Regardless of the temperature during irradiation, the level of fat did not have a significant effect on the D10 value. Salmonellae behaved like E. coli O157:H7 in low-fat beef, but temperature did not have a significant effect when the pathogen was irradiated in high-fat ground beef. Significantly higher D10 values were calculated for C. jejuni irradiated in frozen than in refrigerated low-fat beef. C. jejuni was more resistant to irradiation in low-fat beef than in high-fat beef when treatment was at -17 to -15 degrees C. Regardless of the fat level and temperature during inactivation, these pathogens were highly sensitive to gamma irradiation.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anellis A., Berkowitz D., Kemper D. Comparative resistance of nonsporogenic bacteria to low-temperature gamma irradiation. Appl Microbiol. 1973 Apr;25(4):517–523. doi: 10.1128/am.25.4.517-523.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anellis A., Shattuck E., Morin M., Srisara B., Qvale S., Rowley D. B., Ross E. W., Jr Cryogenic gamma irradiation of prototype pork and chicken and antagonistic effect between Clostridium botulinum types A and B. Appl Environ Microbiol. 1977 Dec;34(6):823–831. doi: 10.1128/aem.34.6.823-831.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DUGGAN D. E., ANDERSON A. W., ELLIKER P. R. INACTIVATION OF THE RADIATION-RESISTANT SPOILAGE BACTERIUM MICROCOCCUS RADIODURANS. II. RADIATION INACTIVATION RATES AS INFLUENCED BY MENSTRUUM TEMPERATURE, PREIRRADIATION HEAT TREATMENT, AND CERTAIN REDUCING AGENTS. Appl Microbiol. 1963 Sep;11:413–417. doi: 10.1128/am.11.5.413-417.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies R., Sinskey A. J. Radiation-resistant mutants of Salmonella typhimurium LT2: development and characterization. J Bacteriol. 1973 Jan;113(1):133–144. doi: 10.1128/jb.113.1.133-144.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farkas J. Microbiological safety of irradiated foods. Int J Food Microbiol. 1989 Aug;9(1):1–15. doi: 10.1016/0168-1605(89)90032-9. [DOI] [PubMed] [Google Scholar]
- STAPLETON G. E. Variations in the sensitivity of escherichia coli to ionizing radiations during the growth cycle. J Bacteriol. 1955 Oct;70(4):357–362. doi: 10.1128/jb.70.4.357-362.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steele J. H., Engel R. E. Radiation processing of food. J Am Vet Med Assoc. 1992 Nov 15;201(10):1522–1529. [PubMed] [Google Scholar]



