Abstract
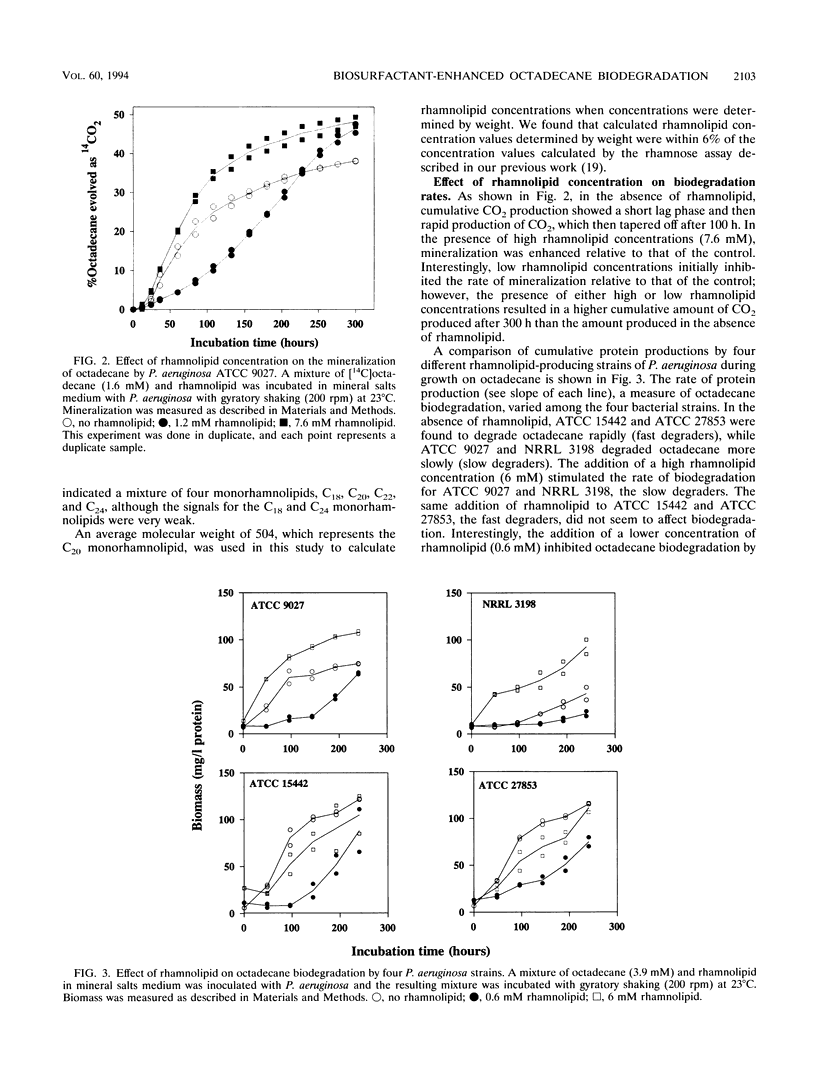
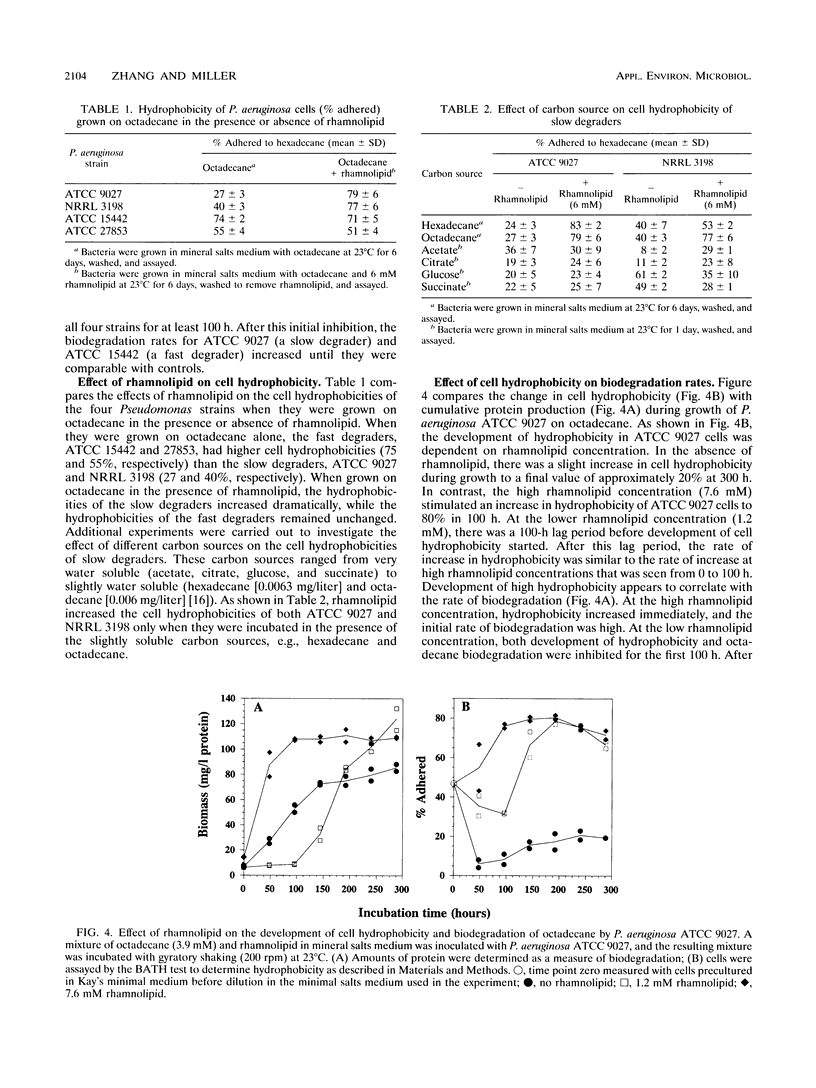
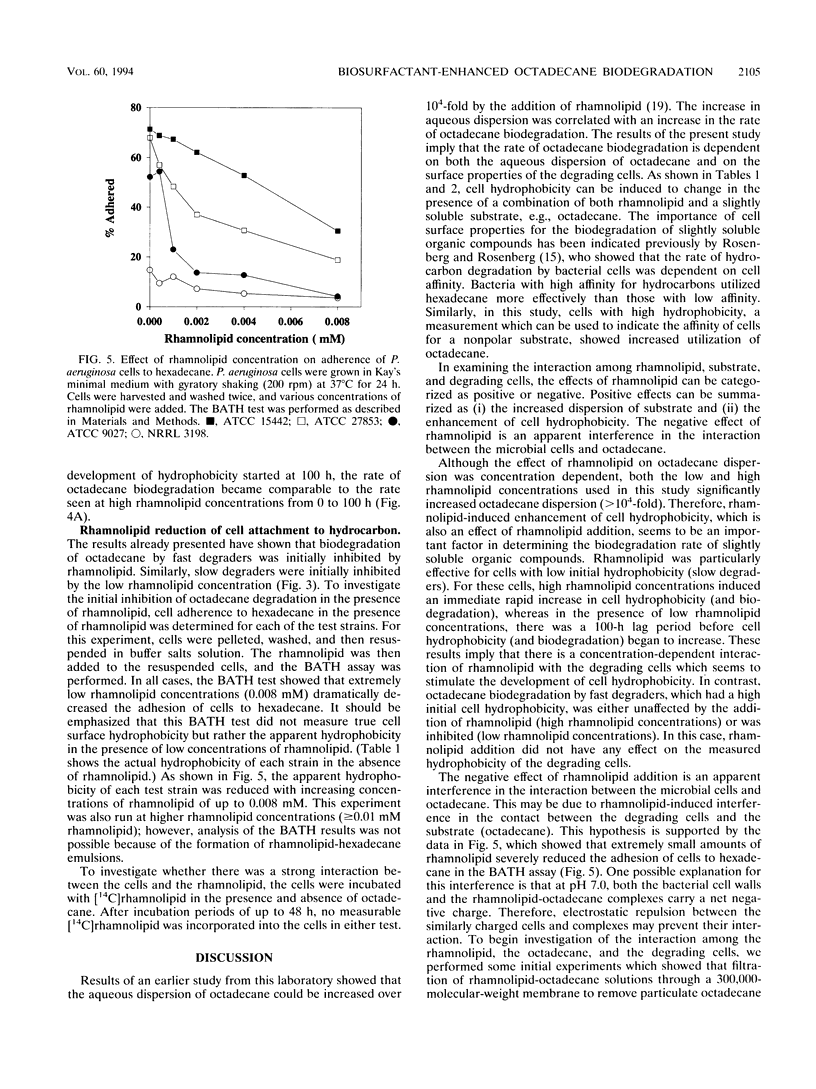
In this study, the effect of a purified rhamnolipid biosurfactant on the hydrophobicity of octadecane-degrading cells was investigated to determine whether differences in rates of octadecane biodegradation resulting from the addition of rhamnolipid to four strains of Pseudomonas aeruginosa could be related to measured differences in hydrophobicity. Cell hydrophobicity was determined by a modified bacterial adherence to hydrocarbon (BATH) assay. Bacterial adherence to hydrocarbon quantitates the preference of cell surfaces for the aqueous phase or the aqueous-hexadecane interface in a two-phase system of water and hexadecane. On the basis of octadecane biodegradation in the absence of rhamnolipid, the four bacterial strains were divided into two groups: the fast degraders (ATCC 15442 and ATCC 27853), which had high cell hydrophobicities (74 and 55% adherence to hexadecane, respectively), and the slow degraders (ATCC 9027 and NRRL 3198), which had low cell hydrophobicities (27 and 40%, respectively). Although in all cases rhamnolipid increased the aqueous dispersion of octadecane at least 10(4)-fold, at low rhamnolipid concentrations (0.6 mM), biodegradation by all four strains was initially inhibited for at least 100 h relative to controls. At high rhamnolipid concentrations (6 mM), biodegradation by the fast degraders was slightly inhibited relative to controls, but the biodegradation by the slow degraders was enhanced relative to controls. Measurement of cell hydrophobicity showed that rhamnolipids increased the cell hydrophobicity of the slow degraders but had no effect on the cell hydrophobicity of the fast degraders. The rate at which the cells became hydrophobic was found to depend on the rhamnolipid concentration and was directly related to the rate of octadecane biodegradation.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cheng K. J., Ingram J. M., Costerton J. W. Release of alkaline phosphatase from cells of Pseudomonas aeruginosa by manipulation of cation concentration and of pH. J Bacteriol. 1970 Nov;104(2):748–753. doi: 10.1128/jb.104.2.748-753.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito S., Inoue S. Sophorolipids from Torulopsis bombicola: possible relation to alkane uptake. Appl Environ Microbiol. 1982 Jun;43(6):1278–1283. doi: 10.1128/aem.43.6.1278-1283.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones N. C., Osborn M. J. Interaction of Salmonella typhimurium with phospholipid vesicles. Incorporation of exogenous lipids into intact cells. J Biol Chem. 1977 Oct 25;252(20):7398–7404. [PubMed] [Google Scholar]
- Käppeli O., Finnerty W. R. Partition of alkane by an extracellular vesicle derived from hexadecane-grown Acinetobacter. J Bacteriol. 1979 Nov;140(2):707–712. doi: 10.1128/jb.140.2.707-712.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Miller R. M., Bartha R. Evidence from liposome encapsulation for transport-limited microbial metabolism of solid alkanes. Appl Environ Microbiol. 1989 Feb;55(2):269–274. doi: 10.1128/aem.55.2.269-274.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberbremer A., Müller-Hurtig R., Wagner F. Effect of the addition of microbial surfactants on hydrocarbon degradation in a soil population in a stirred reactor. Appl Microbiol Biotechnol. 1990 Jan;32(4):485–489. doi: 10.1007/BF00903788. [DOI] [PubMed] [Google Scholar]
- Rendell N. B., Taylor G. W., Somerville M., Todd H., Wilson R., Cole P. J. Characterisation of Pseudomonas rhamnolipids. Biochim Biophys Acta. 1990 Jul 16;1045(2):189–193. doi: 10.1016/0005-2760(90)90150-v. [DOI] [PubMed] [Google Scholar]
- Rosenberg E., Gottlieb A., Rosenberg M. Inhibition of bacterial adherence to hydrocarbons and epithelial cells by emulsan. Infect Immun. 1983 Mar;39(3):1024–1028. doi: 10.1128/iai.39.3.1024-1028.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg M., Rosenberg E. Role of adherence in growth of Acinetobacter calcoaceticus RAG-1 on hexadecane. J Bacteriol. 1981 Oct;148(1):51–57. doi: 10.1128/jb.148.1.51-57.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WARREN R. A., ELLS A. F., CAMPBELL J. J. Endogenous respiration of Pseudomonas aeruginosa. J Bacteriol. 1960 Jun;79:875–879. doi: 10.1128/jb.79.6.875-879.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Miller R. M. Enhanced octadecane dispersion and biodegradation by a Pseudomonas rhamnolipid surfactant (biosurfactant). Appl Environ Microbiol. 1992 Oct;58(10):3276–3282. doi: 10.1128/aem.58.10.3276-3282.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Loosdrecht M. C., Lyklema J., Norde W., Schraa G., Zehnder A. J. The role of bacterial cell wall hydrophobicity in adhesion. Appl Environ Microbiol. 1987 Aug;53(8):1893–1897. doi: 10.1128/aem.53.8.1893-1897.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]



