Abstract
We provide evidence that the tripeptide thiol glutathione (GSH) participates in the regulation of cell division in the apical meristem of Arabidopsis roots. Exogenous application of micromolar concentrations of GSH raised the number of meristematic cells undergoing mitosis, while depletion of GSH had the opposite effect. A role for endogenous GSH in the control of cell proliferation is also provided by mapping of GSH levels in the root meristem using the GSH-specific dye monochlorobimane and confocal laser scanning microscopy. High levels of GSH were associated with the epidermal and cortical initials and markedly lower levels in the quiescent center. The mechanisms controlling cell division could also be triggered by other reducing agents: ascorbic acid and dithiothreitol. Our data also reveal significant plasticity in the relationship between the trichoblast cell length and the hair it subtends in response to alterations in intracellular redox homeostasis. While mechanisms that control trichoblast elongation are influenced by nonspecific redox couples, root hair tip growth has a more specific requirement for sulfhydryl groups. The responses we describe here may represent the extremes of redox control of root plasticity and would allow the root to maintain exploration of the soil under adverse conditions with minimal cell divisions and root hair production or capitalize on a favorable environment by production of numerous long hairs. Redox sensing of the environment and subsequent redox-dependent modulation of growth and development may be crucial components in the strategies plants have evolved for survival in a fluctuating environment.
Keywords: ascorbate, bimane, confocal laser scanning microscopy, glutathione, thiols
The debilitating effects of adverse environmental conditions on plant growth and development are thought to result in part from enhanced formation of active oxygen species. Central to the efficient removal of active oxygen species and the maintenance of cellular redox homeostasis is the ascorbic acid (AA)–glutathione (GSH) cycle, comprised of three interdependent redox couples: AA/dehydroascorbate, GSH/oxidized GSH, and NAD(P)H/NAD(P). Experimental manipulation of the pool sizes or the efficiency of redox cycling of any one of these components has clearly demonstrated their importance in active oxygen species removal (1–3). An important adaptive response of plants to conditions that increase active oxygen species is significant amplification of their antioxidant defenses, and the speed of this response may be critical for stress tolerance (2, 4). This implies that mechanisms exist to sense levels of oxidative stimuli and act to modulate the antioxidant pool size. Indeed plants, as sessile organisms, may be heavily dependent upon such signal transduction pathways for successful growth and reproduction. At present, we have a very limited understanding of how plants sense environmental adversity and how these signal transduction pathways modulate the antioxidant pool size or redox status. Clues are now, however, forthcoming. For example, hydrogen peroxide (H2O2) may act as a signaling molecule, potentially interacting with cytosolic Ca2+ signaling pathways, and the amplitude of this signal may be determined by the GSH pool size or redox status (5). In turn, H2O2 may directly influence GSH synthesis during the onset of oxidative stress (2).
Plants also continuously modify aspects of their developmental program to optimize their morphology to the prevailing local environment. This is most clearly seen for roots, where the growth and configuration of the root system, such as number of lateral roots, is largely determined by environmental factors (6). In this context, the recent demonstration that AA participates as a key regulatory component in the spatial coordination of cell proliferation in the maize root meristem is of considerable interest (7). Given the tightly coordinated relationship between the pools of AA, GSH, and NAD(P)H and their redox status, it is important to establish whether this response is unique to AA (and maize) or reflects a general pattern of redox control of development. In principle, redox intermediates could act both as sensors and effectors to provide a direct link between environmental stress and morphological adaptation through alterations in the patterns of cell division in the primary root apical meristem.
In this study, we investigated the effects of redox agents on cellular proliferation in Arabidopsis roots and the developmental consequences of alterations in cellular redox status. The Arabidopsis root was used as a model dicot system, as it has a simple, well defined three-dimensional organization that reflects a predictable sequence of cell division, expansion, and differentiation (8–10). The roots of 5-day-old Arabidopsis seedlings were exposed to micromolar concentrations of GSH, AA and the synthetic thiol DTT, and the effects of these treatments on root growth and cell division were measured. The effects of GSH depletion through exposure to l-buthionine (S,R)-sulfoximine (BSO), a specific inhibitor of GSH synthesis (11), were also evaluated. BSO treatments not only served to determine the relative contribution of the endogenous GSH pool to root development, but also gave insights into how the endogenous AA pool functions in the absence of GSH. Marked effects of these treatments were also observed on root hair density, trichoblast (hair) cell length, and root hair length. In addition, we mapped GSH levels in situ using noninvasive confocal microscopy to determine the spatial distribution of GSH in the Arabidopsis root meristem.
MATERIALS AND METHODS
Plant Material and Treatments.
Seeds of Arabidopsis thaliana (L.) Heynh ecotype Col-0 were germinated on vertical 0.6% (wt/vol) agarose plates (Murashige and Skoog medium supplemented with 1% sucrose, 100 mg/liter myo-inositol, 500 mg/liter 2-(N-morpholino)ethanesulfonic acid, 500 μg/liter pyridoxine, 1 mg/liter thiamine, and 500 μg/liter nicotinic acid) for 5 days at 21°C under a 16 h light/8 h dark regime. For treatment, seedlings were carefully transferred to plates containing the above medium supplemented with 10 or 100 μM GSH, DTT, and AA or 1 mM BSO (Sigma), and grown for a further 48 h.
Root Length, Hair Length, and Trichoblast Length Measurements After Exposure to Redox Agents.
Upon transfer to the plates containing the various media, the position of the root tip was marked on the underside of the plate. After 24 h, the region of the root that had grown subsequently to transfer was photographed using a camera mounted on a dissecting microscope using a ×20 magnification. The lengths of root hairs were measured, and the number of root hair cells were counted in a 1-mm section of the root where trichoblasts had fully expanded. To measure trichoblast cell length, 40 mature trichoblasts from 10 roots per treatment were examined 48 h after transfer using Normarski optics on a Axioskop microscope (Zeiss) fitted with a 20× Achroplan lens with the aid of an eyepiece graticule. Data were analyzed in excel (Microsoft) using the Student’s one-tailed t test for independent samples (α = 0.05) to determine the significance of differences between treatment means. An f test for homogeneity of variance was performed on data to determine whether a t test for equal or unequal variances should be used. Results are reported as significantly different where 0.001 < P < 0.01 and highly significantly different where P < 0.001.
Dye Loading and Confocal Laser Scanning Microscopy.
GSH levels were visualized in intact roots of Arabidopsis after conjugation with monochlorobimane (Molecular Probes) in situ to give a fluorescent GS-bimane adduct. Seedlings were carefully removed from the agarose surface, and the root was placed in a drop of 10–100 μM monochlorobimane freshly made from a 100 mM stock in ethanol. After labeling for 10–15 min, the root was washed briefly in distilled water and mounted on a microscope slide under a coverslip supported by additional coverslips to prevent squashing the tissue. This preparation was placed on the stage of a Nikon Diaphot inverted microscope attached to a modified Bio-Rad MRC 600 confocal imaging system (12, 13). Optical sections were averaged over three to four frames with excitation at 442 nm and emission at 563 ± 88 nm using a Nikon 10× 0.5 NA Fluor lens, a Zeiss 25× 0.8 NA Plan-NeoFluar multi-immersion lens, or a Nikon 60× 1.4 NA Plan-Apochromat oil immersion lens. Three-dimensional images were collected with a z focus increment of 2–5 μm. Imaging conditions were optimized to maintain the viability of the tissue, according to previously described protocols (14, 15). Total fluorescence levels along the root were measured from intensity profiles taken along average z projections of serial optical sections collected with the ×10 lens. Average fluorescence intensities for the quiescent center (QC) and epidermal/cortical initials were measured from user-defined regions on time course images collected with the 25× lens. Image collection was performed using mpl, comos, and tcsm (Bio-Rad). Statistical and graphical analysis were performed using excel (Microsoft). Image montages were assembled with photoshop (Adobe Systems, Mountain View, CA).
Measurement of Mitotic Index.
To visualize mitotic figures, four roots per treatment were labeled with the DNA-specific stain chromomycin A3 according to Hughes and McCully (16). Serial optical sections were Kalman averaged over three to four frames with excitation at 442 nm and emission at 563 ± 88 nm. The focus increment was 2–5 μm using a Nikon 10× 0.5 NA Fluor lens, a Zeiss 25× 0.8 NA Plan-NeoFluar multi-immersion lens, or a Nikon 60× 1.4 NA Plan-Apochromat oil immersion lens. The number of metaphases and anaphases were counted in the first 250 μm of root from individual optical sections displayed on the computer screen.
RESULTS
Redox Agents Alter Arabidopsis Root Growth.
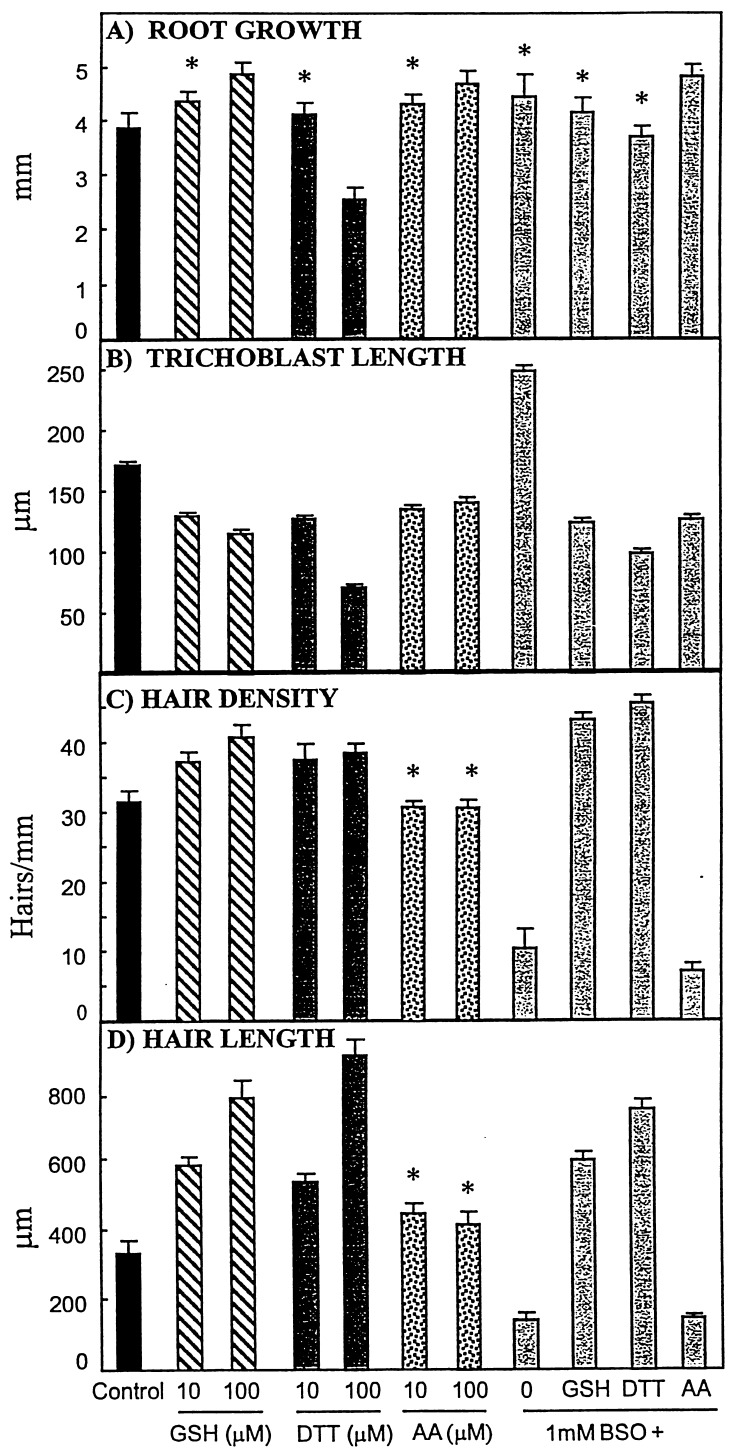
The primary roots of 5-day-old Arabidopsis seedlings increased in length by an average of ≈4 mm over a 24-h period after transfer to control medium (Fig. 1A). The presence of GSH caused a significant, dose-dependent increase in the mean root length, reaching 126% of controls (P < 0.001) at 100 μM concentration, while at the same concentration AA caused an increase of borderline significance (P = 0.02). In contrast, roots only grew to 64% of the mean control length in the presence of the synthetic thiol DTT at the same concentration. Depletion of GSH through BSO treatment did not affect root length and did not alter the stimulation of root length observed with 100 μM GSH and AA (Fig. 1A). In contrast, treatment with 1 mM BSO was sufficient to abolish the decrease in length observed in roots exposed to 100 μM DTT.
Figure 1.
Effect of redox agents on Arabidopsis root morphology. (A) Average root growth measured 24 h after transfer to the different media (n = 10). (B) Average trichoblast length measured on cells that had fully elongated after transfer (n = 40). (C) Average hair density, expressed as the number counted in a 1-mm section of the root in which trichoblasts had fully elongated after transfer (n ≥ 6). (D) Length of root hairs measured in the region of the root in which trichoblasts had fully elongated after transfer (n ≥ 6). Bars represent SEs. Asterisks indicate data that were not significantly different to controls. All other data were significantly (P < 0.01) or highly significantly different (P < 0.001) than controls.
Redox Agents Alter Arabidopsis Trichoblast Length.
To determine whether the redox effects on root growth were mediated by changes in cell number, cell elongation or both, we measured the length of mature, fully expanded trichoblasts. Trichoblasts from control Arabidopsis Col-0 seedlings had a mean length of 170.9 ± 3.7 μm (Fig. 1B), in agreement with the findings of Masucci and Schiefelbein (17). GSH and DTT caused dose-dependent decreases in the mean trichoblast length, only reaching 67% and 41%, respectively, of the mean control length at 100 μM. The effects of AA were less marked (79% of controls), but still highly significant (P < 0.001), and appeared to be maximal even at 10 μM. In contrast, depletion of GSH through BSO treatment caused a highly significant increase in mean trichoblast length to 145% of controls (P < 0.001). The effects of BSO were completely reversed by 100 μM GSH, DTT, or AA, which all still resulted in a reduction in the average trichoblast length.
Effect of Redox on Cell Divisions in the Meristematic Zone.
As the trichoblast length decreased under conditions where the root length was either maintained or even greater than controls, we wished to test whether additional cell divisions were taking place in the meristem in response to redox treatments. The number of mitotic (metaphase and anaphase) figures counted in the terminal 250 μm in control roots was 16 ± 2. In roots exposed to GSH and AA, the number of mitotic figures in the same zone increased (28 ± 1.8 at 100 μM GSH and 20 ± 2 at 10 μM AA), compared with control. Furthermore in roots treated with GSH, the zone of meristematic activity extended further away from the apex into the nominal elongation zone. In roots treated with 1 mM BSO the number of mitotic figures decreased compared with control roots (12 ± 3).
Endogenous GSH Levels in the Meristematic Zone.
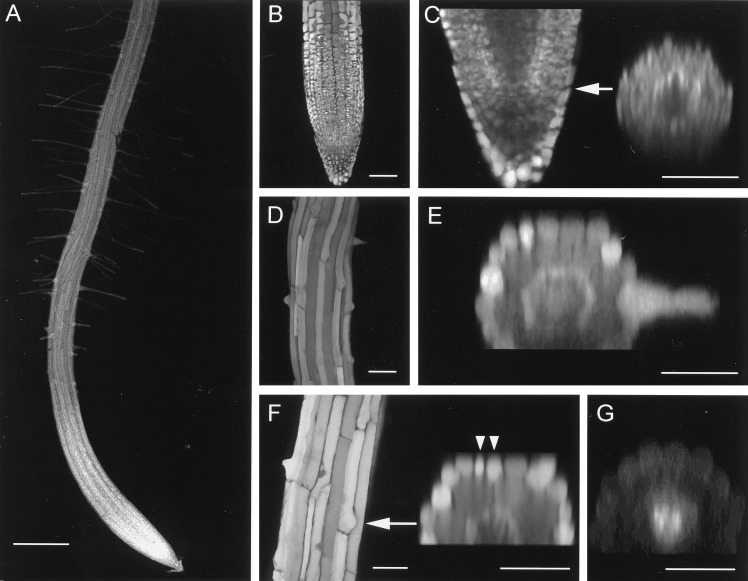
To determine whether different levels of GSH might be associated with cell proliferation during normal development, we measured the three-dimensional distribution of fluorescence after conjugation of endogenous GSH to monochlorobimane in living roots (Fig. 2). GS-bimane fluorescence levels have previously been used as a reporter of GSH in living mammalian tissues (18–20). Our data indicate that GS-bimane is also a faithful reporter for GSH levels in planta (M.J.M., N.S.W., and M.F., unpublished data). Visualization of the three-dimensional images from the live tissue as maximum projections or digital longitudinal or digital cross sections revealed a complex pattern of fluorescence with marked heterogeneity between different cell types. Strong GS-bimane fluorescence was associated with the apical meristem (Fig. 2 A–C) and the trichoblast cell files (Fig. 2 D and E). Generally cells with the largest predicted vacuole to cytoplasm ratio had lower fluorescence levels. Thus, the average intensity decreased as cells in a file elongated (Fig. 2A). Trichoblast cell files had higher average fluorescence than atrichoblast and cortical cell files (Fig. 2 D and E), particularly before root hair initiation. The endodermis was consistently bright (Fig. 2E); however, it was not possible to distinguish clearly details within the pericycle. Of particular interest was the observation that the average GS-bimane fluorescence from the QC was ≈27% lower (0.73 ± 0.06, n = 5) than the neighboring epidermal and cortical initial cells (Fig. 2C). Treatment with 10 μM GSH increased the fluorescence in all cell types (Fig. 2F). The average increase in fluorescence over a 1200-μm length of root was 180% compared with controls. In this root, there was also a bifurcation of the trichoblast cell file (Fig. 2F, arrowheads) possibly arising through an altered plane of cell division. Very occasionally, ectopic trichoblast cell files were seen with GSH and DTT treatment (data not shown). Staining of roots pretreated with BSO for 24 h revealed that, in nearly all cell types, fluorescence was reduced to <5% of controls, apart from two regions in the vascular tissue (Fig. 2G). In untreated tissues (Fig. 2E) or in roots treated with GSH (Fig. 2F), only low levels of fluorescence were present in this region.
Figure 2.
Visualization of GS-bimane conjugates in intact roots of Arabidopsis Col-0 seedlings in three-dimensional images collected using confocal laser scanning microscopy. Intact Arabidopsis roots were labeled for 10–15 min with 100 μM monochlorobimane, and serial optical sections were collected at 3 μm focus increments with excitation at 442 nm and emission at 563 ± 87 nm. (A) Low magnification extended focus fluorescence image using a ×5 lens. (B) Maximum projection from 40 optical longitudinal (xy) sections of GS-bimane fluorescence at the root tip. (C) Single optical longitudinal section from B through the midplane of the root and the corresponding single digital cross (xz) section through the QC at the position marked. Note the reduced GS-bimane labeling in the QC compared with the neighboring cortical and epidermal initials. The decrease in fluorescence toward the base of the xz section arises from specimen- and depth-dependent signal attenuation. (D) Maximum projection from 40 optical longitudinal sections of GS-bimane fluorescence in the region of root hair initiation. (E) Single digital cross section from D. Note the increased fluorescence in the trichoblast cell files. The root continued to grow during the imaging period. (F) Maximum projection and single xz section of GS-bimane fluorescence in the region of root hair initiation of a root treated with 10 μM GSH for 24 h. Note the increase in overall fluorescence compared with that in D and E and the bifurcation of the trichoblast cell file. (G) xz section of a root treated with 1 mM BSO for 24 h, then stained with monochlorobimane for 15 min. Labeling is limited to two regions within the vascular tissue that may represent GSH in the phloem. The intensity has been scaled by a factor of 2 compared with the other images. [Bars = 250 μm (A) and 50 μm (B–G).]
Effect of Redox on Root Hair Density.
The mean hair density of control roots after transfer was 31.6 ± 1.6 per mm. GSH and DTT caused highly significant, dose-dependent increases in hair density (P < 0.001), whereas AA was without any significant effect, even at 100 μM (Fig. 1C). BSO dramatically reduced the hair density to 33% of controls through a combined effect of longer trichoblast cells (Fig. 1B) and the fact that hairs did not initiate on all trichoblasts in a file (data not shown). This effect was completely reversed by 100 μM GSH and DTT, which both resulted in a significant increase in hair density over controls, similar to results in the absence of BSO. In contrast, addition of 100 μM AA was not able to reverse the effect of GSH depletion through BSO treatment (Fig. 1C).
Thiols Stimulate Arabidopsis Root Hair Elongation.
Treatments with GSH and DTT also caused a highly significant, dose-dependent increase in root hair length, reaching 234% and 271% of controls, respectively, at 100 μM (Fig. 1D). In contrast, AA had no significant effect on hair length, even at 100 μM. Neither thiols or AA affected the lengths of root hairs that had initiated before transfer (data not shown). Root hair lengths in treatments with BSO were only 42% of controls. Both GSH and DTT completely reversed the effect of BSO on hair length; however, AA did not (Fig. 1D).
DISCUSSION
A characteristic feature of plants with a sessile lifestyle in a fluctuating environment is a plastic developmental program that is responsive to external signals. Molecules that undergo reversible alterations in response to environmental change are ideal candidates to act as environmental sensors. A role for the interdependent redox couples of the AA–GSH cycle (AA/dehydroascorbate, GSH/oxidized GSH, and NAD(P)H/NAD(P)) is now well established in adaptation to environmental adversity and oxidative stress (1–3). More recently, AA was implicated in the regulation of cell proliferation in the root meristem of maize, opening the possibility that redox state might directly couple the developmental program to the level of environmental stress (7).
Here we present evidence that GSH participates in the regulation of cell division in the primary meristem of Arabidopsis roots. This is inferred from three lines of evidence: first, artificially increased endogenous GSH levels stimulated cell divisions; second, artificially decreasing GSH levels through BSO treatment reduced cell divisions; and third, high levels of endogenous GSH were associated with actively dividing initial cells, but not the slowly cycling cells of the QC. The mechanisms controlling cell division at the root apex in Arabidopsis were not specific for GSH, but could also be triggered with other exogenous, diffusible redox agents such as DTT and also AA, as previously described (7). Importantly, the inhibition of cell division through BSO treatment could be reversed by supplementing either GSH, DTT, or AA, indicating that BSO acts through depletion of GSH, and not some unknown toxic effect. A role for endogenous GSH in the control of cell proliferation in roots can also be inferred from the distribution of GS-bimane fluorescence. Fluorescence was highest in regions of cellular proliferation, such as the epidermal and cortical initials, but reduced in several other cell types, most notably the slowly cycling cells of the QC in the root apex, which have an extended G1 phase. This distribution closely parallels that described for the pattern of AA in the maize apical root meristem (7). It is possible that the differences in the level of GSH in the QC and surrounding initials in the apical meristem of Arabidopsis roots could simply reflect differences in metabolic activity between the different cell types. Nevertheless, given the strong correlation of the precise pattern of GS-bimane fluorescence and cell function within the root meristem, mechanisms must operate to maintain this spatial heterogeneity. For comparison, in the maize QC, quiescence appears to be maintained by active elimination of AA (7). Our data also indicate that mechanisms may normally operate to lower the GSH and AA pools or shift their redox balance in cells distant to the meristem initials to restrict the extent of the meristematic zone. Indeed, exogenous application of GSH was found to promote divisions at some distance from the apex. Thus the redox state of low-molecular weight antioxidants may modulate the degree of proliferation and its integration into the developmental program, with consequences for subsequent morphogenesis. The observations, implicating a causal link between the levels of GSH and/or AA and the cell cycle in plants, also parallel recent results from other systems. The cellular redox state, in particular the level of GSH, is a critical factor in modulating the activity of transcription factors responsible for the G1/S phase transition in animals (21). In turn, the redox state of the cell at the G1/S transition may be determined by de novo synthesis of GSH and the level of GSH may directly modulate the synthesis of DNA after mitogenic stimulation (22, 23). Thus redox regulation of cellular proliferation may share common elements between higher eukaryotes, indicating strong selective pressure for evolutionary conservation.
Changes in rates of cell divisions in the meristem may alter cell number; however, the consequences for root length depend on the amount of subsequent cell elongation. Thus, in GSH- and AA-treated tissues, the product of more, but shorter trichoblast cells was sufficient to give an increase in overall root length. The synthetic thiol DTT had similar effects at 10 μM, although at 100 μM there was a marked reduction in root growth. In general, the effects of DTT were more pronounced than GSH or AA at the same concentrations, as might be anticipated for a nonmetabolizable thiol reagent. In BSO-treated tissues, cell division was reduced compared with controls; however, root length was maintained at the control value by an increase in cell elongation. At this stage, it is not clear whether the reduction in trichoblast length as a result of increased GSH or AA levels can be attributed solely to partitioning into smaller units with increased cell divisions or via specific effects on mechanisms regulating cell elongation or via nonspecific effects, such as modulating the crosslinking of cell wall proteins (24).
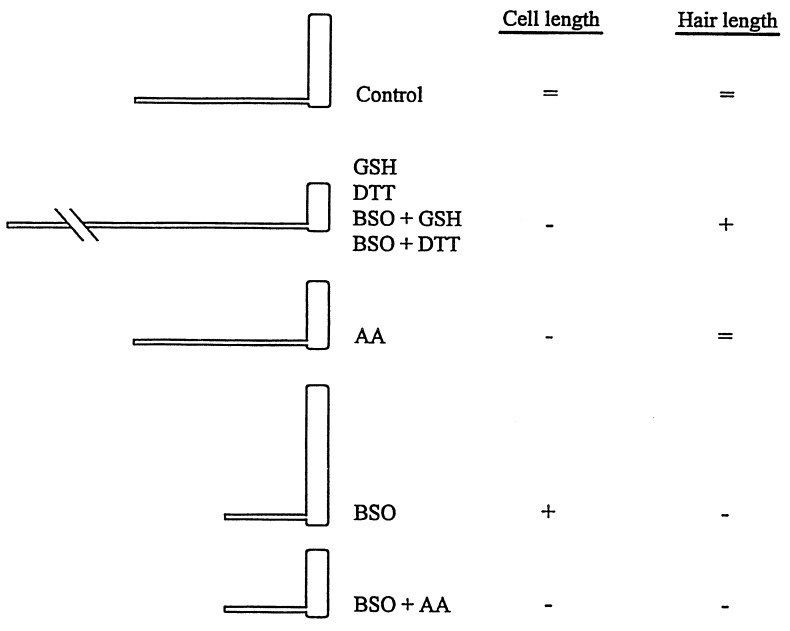
Our data also highlighted significant plasticity in the relationship between the size of the trichoblast cell and the hair it subtends in response to alterations in the intracellular redox homeostasis. In wild-type Arabidopsis roots, hairs emerge from the apical end of the trichoblast cell once elongation has ceased (12), and there is a consistent relationship between the size of the trichoblast and the length of the hair for a given Arabidopsis ecotype (17). Modification of the cellular redox status can substantially alter this relationship (Fig. 3). In general, there appears to be an inverse relationship between trichoblast length and hair length. Addition of GSH and DTT produced short trichoblasts with long hairs, whereas depletion of GSH led to long trichoblasts with short hairs compared with controls, and in some cases, the hair did not initiate at all, as was demonstrated by the lower root hair density of BSO-treated roots. This might be ascribed to simple partitioning of resources between the two structures. However, our data indicate that within this overall trend, high levels of thiols may specifically enhance hair tip growth and this effect cannot be mimicked by AA (Fig. 3). Furthermore, while GSH, DTT, and AA could reverse the effects of BSO on trichoblast elongation, the effects of BSO on hair elongation could only be reversed by thiols (Fig. 3). Our data indicate that while mechanisms that control trichoblast elongation are influenced simply by a more reducing environment provided by nonspecific redox couples, root hair tip growth may have a more specific requirement for redox properties specific to sulfhydryl groups. A wide range of factors are known to influence Arabidopsis root hair tip growth, including auxin, calcium, pH, light, phosphorylation, and nutrients (6). How these diverse influences are coordinated to affect hair growth is not known, but clearly, as we have shown, the redox state of cellular thiols play a key role in this process. The characteristic relationship between the length of the trichoblast, the hair density, and the hair length in the wild-type root under controlled growth conditions would suggest that the cellular redox environment is maintained at a particular set point to generate the stereotyped morphology of the root. It will be interesting to determine the intracellular antioxidant levels and the effects of exogenous redox agents on the morphology of Arabidopsis mutants with altered trichoblast length/hair length ratios, such as the rhd6 mutant (17). Sensitivity of the redox status and pool sizes of GSH and AA in response to environmental fluctuations are potentially important factors in modulating plant development and may represent an important adaptive response to adverse environmental conditions. The responses we describe may represent the extremes of redox control of root plasticity and would allow the root to maintain exploration of the soil under adverse conditions with minimal cell divisions and root hair production or capitalize on a favorable local environment by production of numerous long root hairs. Redox sensing of the environment and subsequent redox-dependent modulation of growth and development may be crucial components in the adaptive strategies plants have evolved for survival in a patchy and constantly fluctuating environment.
Figure 3.
Schematic representation of the effects of redox agents on trichoblast length and root hair length. =, Control length or not significantly different from control; +, significantly longer than control; and −, significantly shorter than control.
Acknowledgments
We thank Tom Gerats, Téva Vernoux, and Rachel Errington for comments on the work and critical reading of the manuscript. This work was supported by grants from Belgian Programme on Interuniversity Poles of Attraction (Prime Minister’s Office, Science Policy Programming; Grant 38), the Vlaams Actieprogramma Biotechnologie (ETC 002), the INTAS Project (94-774), and the International Atomic Energy Agency (5285). R.S.-F. is indebted to the Dirección General de Investigación Científica y Técnica (Ministerio de Educación y Ciencia, Spain); M.J.M. to Glasstone Fellowship, to the Ministry of Agriculture, Fisheries and Food (United Kingdom), and to the European Molecular Biology Organization for postdoctoral fellowships; and L.C.B. to a Human Capital and Mobility Fellowship (ERBCHBGCT92-0097).
ABBREVIATIONS
- AA
ascorbic acid
- GSH
glutathione
- BSO
l-buthionine (S,R)-sulfoximine
- QC
quiescent center
References
- 1.Conklin P L, Williams E H, Last R L. Proc Natl Acad Sci USA. 1996;93:9970–9974. doi: 10.1073/pnas.93.18.9970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.May M J, Leaver C J. Plant Physiol. 1993;103:621–627. doi: 10.1104/pp.103.2.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Babiychuk E, Kushnir S, Belles-Boix E, Van Montagu M, Inzé D. J Biol Chem. 1995;270:26224–26231. doi: 10.1074/jbc.270.44.26224. [DOI] [PubMed] [Google Scholar]
- 4.Bowler C, Van Montagu M, Inzé D. Annu Rev Plant Physiol Plant Mol Biol. 1992;43:83–116. [Google Scholar]
- 5.Price A H, Taylor A, Ripley S J, Griffiths A, Trewavas A J, Knight M R. Plant Cell. 1994;6:1301–1310. doi: 10.1105/tpc.6.9.1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aeschbacher R A, Schiefelbein J W, Benfey P N. Annu Rev Plant Physiol Plant Mol Biol. 1994;45:25–45. [Google Scholar]
- 7.Kerk N M, Feldmann L J. Development (Cambridge, UK) 1995;121:2825–2833. [Google Scholar]
- 8.Dolan L, Janmaat K, Willemsen V, Linstead P, Poethig S, Roberts K, Scheres B. Development (Cambridge, UK) 1993;119:71–84. doi: 10.1242/dev.119.1.71. [DOI] [PubMed] [Google Scholar]
- 9.Dolan L, Duckett C M, Grierson C, Linstead P, Schneider K, Lawson E, Dean C, Poethig S, Roberts K. Development (Cambridge, UK) 1994;120:2465–2474. [Google Scholar]
- 10.Scheres B, Wolkenfelt H, Willemsen V, Terlouw M, Lawson E, Dean C, Weisbeek P. Development (Cambridge, UK) 1994;120:2475–2487. [Google Scholar]
- 11.Griffith O, Meister A. J Biol Chem. 1979;254:7558–7560. [PubMed] [Google Scholar]
- 12.Fricker M D, White N S. J Microsc (Oxford) 1992;166:29–42. [Google Scholar]
- 13.Fricker M D, Tlalka M, Ermantraut J, Obermeyer G, Dewey M, Gurr S, Patrick J, White N S. Scanning Microsc Suppl. 1994;8:391–405. [Google Scholar]
- 14.Errington, R. J., Fricker, M. D., Wood, J. L., Hall, A. C. & White, N. S. (1997) Am. J. Physiol., in press. [DOI] [PubMed]
- 15.Fricker M D, Errington R J, Wood J L, Tlalka M, May M, White N S. In: Signal Transduction–Single Cell Research. Van Duijn D, Wiltink A, editors. Heidelberg, Germany: Springer; 1996. in press. [Google Scholar]
- 16.Hughes R, McCully L. Stain Technol. 1975;50:319–329. doi: 10.3109/10520297509117082. [DOI] [PubMed] [Google Scholar]
- 17.Masucci J D, Schiefelbein J W. Plant Physiol. 1994;106:1335–1346. doi: 10.1104/pp.106.4.1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Briviba K, Fraser G, Sies H, Ketterer B. Biochem J. 1993;294:631–633. doi: 10.1042/bj2940631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shrieve D C, Bump E A, Rice G C. J Biol Chem. 1988;263:14107–14114. [PubMed] [Google Scholar]
- 20.Fernández-Checa J, Kaplowitz N. Anal Biochem. 1990;190:212–219. doi: 10.1016/0003-2697(90)90183-a. [DOI] [PubMed] [Google Scholar]
- 21.Russo T, Zambrano N, Esposito F, Ammendola R, Cimino F, Fiscella M, Jackman J, O’Connor M, Anderson C W, Apella E. J Biol Chem. 1995;270:29386–29391. doi: 10.1074/jbc.270.49.29386. [DOI] [PubMed] [Google Scholar]
- 22.Poot M, Teubert H, Rabinovitch P S, Kavanagh T J. J Cell Physiol. 1995;163:555–560. doi: 10.1002/jcp.1041630316. [DOI] [PubMed] [Google Scholar]
- 23.Suthantiran M, Anderson M E, Sharma V K, Meister A. Proc Natl Acad Sci USA. 1990;87:3343–3347. doi: 10.1073/pnas.87.9.3343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bradley D J, Kjellbom P, Lamb C J. Cell. 1992;70:21–30. doi: 10.1016/0092-8674(92)90530-p. [DOI] [PubMed] [Google Scholar]