Abstract
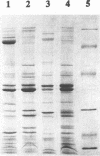
The iron-sequestering abilities of 51 strains of Shewanella putrefaciens isolated from different sources (fish, water, and warm-blooded animals) were assessed. Thirty strains (60%) produced siderophores in heat-sterilized fish juice as determined by the chrome-azurol-S assay. All cultures were negative for the catechol-type siderophore, whereas 24 of the 30 siderophore-producing strains tested positive in the Csáky test, indicating the production of siderophores of the hydroxamate type. Siderophore-producing S. putrefaciens could to some degree cross-feed on the siderophores of other S. putrefaciens strains and on compounds produced by an Aeromonas salmonicida strain under iron-limited conditions. The siderophores of S. putrefaciens were not sufficiently strong to inhibit growth of other bacteria under iron-restricted conditions. However, siderophore-producing Pseudomonas bacteria were always inhibitory to S. putrefaciens under iron-limited conditions. Growth of siderophore-producing strains under iron-limited conditions induced the formation of one major new outer membrane protein of approximately 72 kDa. Two outer membrane proteins of approximately 53 and 23 kDa were not seen when iron was restricted.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amaro C., Aznar R., Alcaide E., Lemos M. L. Iron-binding compounds and related outer membrane proteins in Vibrio cholerae non-O1 strains from aquatic environments. Appl Environ Microbiol. 1990 Aug;56(8):2410–2416. doi: 10.1128/aem.56.8.2410-2416.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkin C. L., Neilands J. B., Phaff H. J. Rhodotorulic acid from species of Leucosporidium, Rhodosporidium, Rhodotorula, Sporidiobolus, and Sporobolomyces, and a new alanine-containing ferrichrome from Cryptococcus melibiosum. J Bacteriol. 1970 Sep;103(3):722–733. doi: 10.1128/jb.103.3.722-733.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aznar R., Alcaide E. Siderophores and related outer membrane proteins produced by pseudomonads isolated from eels and freshwater. FEMS Microbiol Lett. 1992 Nov 1;77(1-3):269–275. doi: 10.1016/0378-1097(92)90168-n. [DOI] [PubMed] [Google Scholar]
- Aznar R., Amaro C., Alcaide E., Lemos M. L. Siderophore production by environmental strains of Salmonella species. FEMS Microbiol Lett. 1989 Jan 1;48(1):7–12. doi: 10.1016/0378-1097(89)90137-7. [DOI] [PubMed] [Google Scholar]
- Chart H., Trust T. J. Acquisition of iron by Aeromonas salmonicida. J Bacteriol. 1983 Nov;156(2):758–764. doi: 10.1128/jb.156.2.758-764.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crosa J. H. A plasmid associated with virulence in the marine fish pathogen Vibrio anguillarum specifies an iron-sequestering system. Nature. 1980 Apr 10;284(5756):566–568. doi: 10.1038/284566a0. [DOI] [PubMed] [Google Scholar]
- Crosa J. H. The relationship of plasmid-mediated iron transport and bacterial virulence. Annu Rev Microbiol. 1984;38:69–89. doi: 10.1146/annurev.mi.38.100184.000441. [DOI] [PubMed] [Google Scholar]
- DiChristina T. J., DeLong E. F. Isolation of anaerobic respiratory mutants of Shewannella putrefaciens and genetic analysis of mutants deficient in anaerobic growth on Fe3+. J Bacteriol. 1994 Mar;176(5):1468–1474. doi: 10.1128/jb.176.5.1468-1474.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filip C., Fletcher G., Wulff J. L., Earhart C. F. Solubilization of the cytoplasmic membrane of Escherichia coli by the ionic detergent sodium-lauryl sarcosinate. J Bacteriol. 1973 Sep;115(3):717–722. doi: 10.1128/jb.115.3.717-722.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill C. O., Newton K. G. Spoilage of vacuum-packaged dark, firm, dry meat at chill temperatures. Appl Environ Microbiol. 1979 Mar;37(3):362–364. doi: 10.1128/aem.37.3.362-364.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gram L. Inhibitory effect against pathogenic and spoilage bacteria of Pseudomonas strains isolated from spoiled and fresh fish. Appl Environ Microbiol. 1993 Jul;59(7):2197–2203. doi: 10.1128/aem.59.7.2197-2203.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gram L., Wedell-Neergaard C., Huss H. H. The bacteriology of fresh and spoiling Lake Victorian Nile perch (Lates niloticus). Int J Food Microbiol. 1990 May;10(3-4):303–316. doi: 10.1016/0168-1605(90)90077-i. [DOI] [PubMed] [Google Scholar]
- Hirst I. D., Hastings T. S., Ellis A. E. Siderophore production by Aeromonas salmonicida. J Gen Microbiol. 1991 May;137(5):1185–1192. doi: 10.1099/00221287-137-5-1185. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lovley D. R. Dissimilatory Fe(III) and Mn(IV) reduction. Microbiol Rev. 1991 Jun;55(2):259–287. doi: 10.1128/mr.55.2.259-287.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massad G., Arceneaux J. E., Byers B. R. Acquisition of iron from host sources by mesophilic Aeromonas species. J Gen Microbiol. 1991 Feb;137(2):237–241. doi: 10.1099/00221287-137-2-237. [DOI] [PubMed] [Google Scholar]
- Myers C. R., Nealson K. H. Respiration-linked proton translocation coupled to anaerobic reduction of manganese(IV) and iron(III) in Shewanella putrefaciens MR-1. J Bacteriol. 1990 Nov;172(11):6232–6238. doi: 10.1128/jb.172.11.6232-6238.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neilands J. B. Microbial iron compounds. Annu Rev Biochem. 1981;50:715–731. doi: 10.1146/annurev.bi.50.070181.003435. [DOI] [PubMed] [Google Scholar]
- Ong S. A., Neilands J. B. Siderophores in microbially processed cheese. J Agric Food Chem. 1979 Sep-Oct;27(5):990–995. doi: 10.1021/jf60225a033. [DOI] [PubMed] [Google Scholar]
- Richard C., Kiredjian M., Guilvout I. Caractères des phénotypes de Alteromonas putrefaciens. Etude de 123 souches. Ann Biol Clin (Paris) 1985;43(5):732–738. [PubMed] [Google Scholar]
- Rogers H. J. Iron-Binding Catechols and Virulence in Escherichia coli. Infect Immun. 1973 Mar;7(3):445–456. doi: 10.1128/iai.7.3.445-456.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwyn B., Neilands J. B. Universal chemical assay for the detection and determination of siderophores. Anal Biochem. 1987 Jan;160(1):47–56. doi: 10.1016/0003-2697(87)90612-9. [DOI] [PubMed] [Google Scholar]
- Shanahan P., O'sullivan D. J., Simpson P., Glennon J. D., O'gara F. Isolation of 2,4-diacetylphloroglucinol from a fluorescent pseudomonad and investigation of physiological parameters influencing its production. Appl Environ Microbiol. 1992 Jan;58(1):353–358. doi: 10.1128/aem.58.1.353-358.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigel S. P., Payne S. M. Effect of iron limitation on growth, siderophore production, and expression of outer membrane proteins of Vibrio cholerae. J Bacteriol. 1982 Apr;150(1):148–155. doi: 10.1128/jb.150.1.148-155.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenström I. M., Molin G. Classification of the spoilage flora of fish, with special reference to Shewanella putrefaciens. J Appl Bacteriol. 1990 Jun;68(6):601–618. doi: 10.1111/j.1365-2672.1990.tb05226.x. [DOI] [PubMed] [Google Scholar]