Abstract
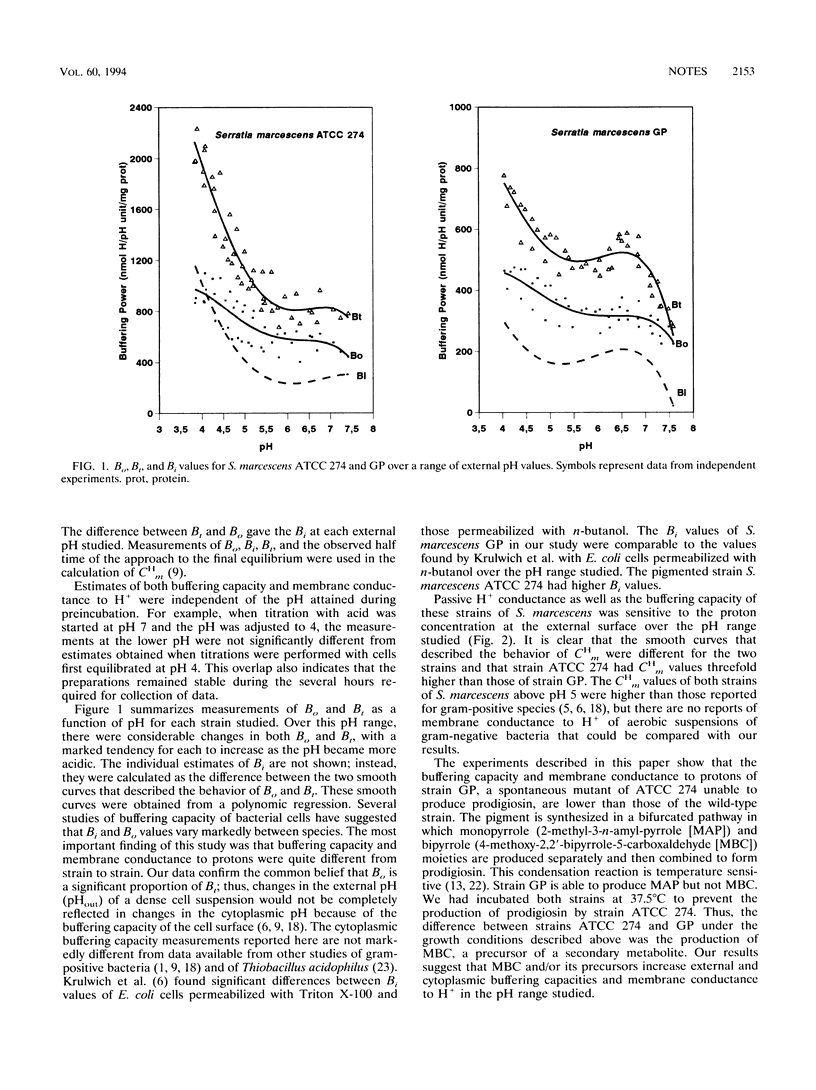
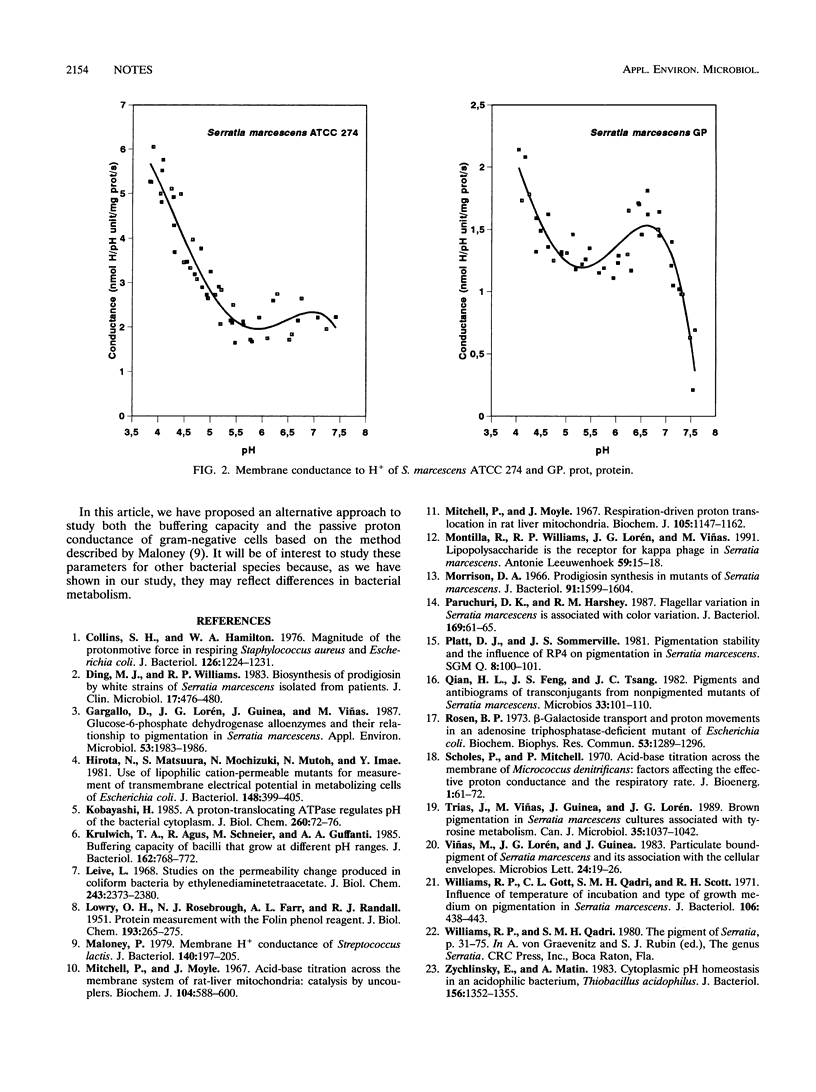
The pigmented strain Serratia marcescens ATCC 274 had a higher buffering capacity and a higher membrane H+ conductance than S. marcescens GP, a spontaneous nonpigmented mutant of ATCC 274. The data suggest that mutations which apparently affect only the synthesis of a secondary metabolite can modify buffering capacity and passive H+ conductance.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Collins S. H., Hamilton W. A. Magnitude of the protonmotive force in respiring Staphylococcus aureus and Escherichia coli. J Bacteriol. 1976 Jun;126(3):1224–1231. doi: 10.1128/jb.126.3.1224-1231.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding M. J., Williams R. P. Biosynthesis of prodigiosin by white strains of Serratia marcescens isolated from patients. J Clin Microbiol. 1983 Mar;17(3):476–480. doi: 10.1128/jcm.17.3.476-480.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gargallo D., Lorén J. G., Guinea J., Viñas M. Glucose-6-phosphate dehydrogenase alloenzymes and their relationship to pigmentation in Serratia marcescens. Appl Environ Microbiol. 1987 Aug;53(8):1983–1986. doi: 10.1128/aem.53.8.1983-1986.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirota N., Matsuura S., Mochizuki N., Mutoh N., Imae Y. Use of lipophilic cation-permeable mutants for measurement of transmembrane electrical potential in metabolizing cells of Escherichia coli. J Bacteriol. 1981 Nov;148(2):399–405. doi: 10.1128/jb.148.2.399-405.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi H. A proton-translocating ATPase regulates pH of the bacterial cytoplasm. J Biol Chem. 1985 Jan 10;260(1):72–76. [PubMed] [Google Scholar]
- Krulwich T. A., Agus R., Schneier M., Guffanti A. A. Buffering capacity of bacilli that grow at different pH ranges. J Bacteriol. 1985 May;162(2):768–772. doi: 10.1128/jb.162.2.768-772.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Leive L. Studies on the permeability change produced in coliform bacteria by ethylenediaminetetraacetate. J Biol Chem. 1968 May 10;243(9):2373–2380. [PubMed] [Google Scholar]
- Maloney P. C. Membrane H+ conductance of Streptococcus lactis. J Bacteriol. 1979 Oct;140(1):197–205. doi: 10.1128/jb.140.1.197-205.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell P., Moyle J. Acid-base titration across the membrane system of rat-liver mitochondria. Catalysis by uncouplers. Biochem J. 1967 Aug;104(2):588–600. doi: 10.1042/bj1040588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell P., Moyle J. Respiration-driven proton translocation in rat liver mitochondria. Biochem J. 1967 Dec;105(3):1147–1162. doi: 10.1042/bj1051147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montilla R., Williams R. P., Lorén J. G., Viñas M. Lipopolysaccharide is the receptor for kappa phage in Serratia marcescens. Antonie Van Leeuwenhoek. 1991 Jan;59(1):15–18. doi: 10.1007/BF00582114. [DOI] [PubMed] [Google Scholar]
- Morrison D. A. Prodigiosin synthesis in mutants of Serratia marcesens. J Bacteriol. 1966 Apr;91(4):1599–1604. doi: 10.1128/jb.91.4.1599-1604.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paruchuri D. K., Harshey R. M. Flagellar variation in Serratia marcescens is associated with color variation. J Bacteriol. 1987 Jan;169(1):61–65. doi: 10.1128/jb.169.1.61-65.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian H. L., Feng J. S., Tsang J. C. Pigments and antibiogram of transconjugants from nonpigmented mutants of Serratia marcescens. Microbios. 1982;33(132):101–110. [PubMed] [Google Scholar]
- Rosen B. P. Beta-galactoside transport and proton movements in an adenosine triphosphatase-deficient mutant of Escherichia coli. Biochem Biophys Res Commun. 1973 Aug 21;53(4):1289–1296. doi: 10.1016/0006-291x(73)90605-0. [DOI] [PubMed] [Google Scholar]
- Scholes P., Mitchell P. Acid-base titration across the plasma membrane of Micrococcus denitrificans: factors affecting the effective proton conductance and the respiratory rate. J Bioenerg. 1970 Jun;1(1):61–72. doi: 10.1007/BF01516089. [DOI] [PubMed] [Google Scholar]
- Trias J., Viñas M., Guinea J., Lorèn J. G. Brown pigmentation in Serratia marcescens cultures associated with tyrosine metabolism. Can J Microbiol. 1989 Nov;35(11):1037–1042. doi: 10.1139/m89-172. [DOI] [PubMed] [Google Scholar]
- Williams R. P., Gott C. L., Qadri S. M., Scott R. H. Influence of temperature of incubation and type of growth medium on pigmentation in Serratia marcescens. J Bacteriol. 1971 May;106(2):438–443. doi: 10.1128/jb.106.2.438-443.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zychlinsky E., Matin A. Cytoplasmic pH homeostasis in an acidophilic bacterium, Thiobacillus acidophilus. J Bacteriol. 1983 Dec;156(3):1352–1355. doi: 10.1128/jb.156.3.1352-1355.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]



