Abstract
Ethylene inhibits hypocotyl elongation in etiolated Arabidopsis seedlings. However, when Arabidopsis was grown in the light in the presence of ethylene or its precursor 1-aminocyclopropane-1-carboxylic acid (ACC), a marked induction of hypocotyl elongation occurred. This resulted from an increase in cell expansion rather than cell division. The effects of ethylene and ACC were antagonized by the ethylene action inhibitor Ag+. The elongation response was absent or weakened in a set of ethylene-insensitive mutants (etr1-3, ein2-1, ein3-1, ein4, ain1-10, ein7). With the exception of ein4, the degree of inhibition of hypocotyl elongation was correlated with the strength of the ethylene-insensitive phenotype based on the triple response assay. In addition, the constitutive ethylene response mutant ctr1-1, grown in the light, had a longer hypocotyl than the wild type. Exogenous auxin also induced hypocotyl elongation in light-grown Arabidopsis. Again, the response was abolished by treatment with Ag+, suggesting that ethylene might be a mediator. The results showed that, depending on light conditions, ethylene can induce opposite effects on cell expansion in Arabidopsis hypocotyls.
Keywords: 1-aminocyclopropane-1-carboxylic acid, auxin, cell expansion, mutant, nutrient deficiency
Plant cell expansion is thought to be controlled by the orientation of cortical microtubules in combination with both the extensibility of the cell wall and the turgor pressure inside the cell (1, 2). These processes are under control of light and phytohormones. Ethylene can both promote and inhibit cell growth depending on plant species and cell type (3). In Arabidopsis, it was found to inhibit cell expansion (4, 5). Hence, Arabidopsis plants treated with ethylene, as well as a mutant displaying constitutive ethylene responses (ctr1), show a severe growth inhibition throughout development. Roots and inflorescences are short and leaves remain unexpanded. In etiolated Arabidopsis seedlings, ethylene prevents hypocotyl elongation (6). Recently, with the cloning and characterization of the Arabidopsis HOOKLESS1 (HLS1) gene, it has been shown that ethylene can also promote cell elongation (7). Specific cells in the apical hook of etiolated seedlings are induced to elongate with differential growth and hook curvature as a result. HLS1 is thought to control growth via regulation of transport or chemical modification of auxin. Other examples of a cross-talk between the ethylene and auxin pathways have been found. Most auxin-resistant mutants appeared to be also ethylene insensitive (8). Moreover, studies with aux1 suggested that ethylene sensitivity is regulated by auxin (9). Current molecular-genetic approaches shed a new light on previous physiological evidence for a complex interplay between these two hormones (10, 11).
Most ethylene mutants in Arabidopsis have been identified using the triple response (12). Analysis of the ethylene-insensitive mutants etr1 (6), ein2 (13), and ain1 (14) demonstrated that the effects of this class of mutations are not restricted to the etiolated seedling stage, but are also observed throughout the life cycle. In contrast, some of the ethylene-overproducing mutants have a markedly higher biosynthesis in the dark, whereas in the light ethylene production is close to or identical with the wild-type level (4, 13). In addition, a mutant was isolated displaying ethylene insensitivity specifically in the roots (eir1) (15). Etiolated seedlings of eir1 have wild-type ethylene responses. This indicates the existence of genes that regulate ethylene biosynthesis or signal transduction at specific stages in development. Screening for ethylene mutants at a developmental stage other than the etiolated seedling stage might allow identification of novel genes involved in the control of ethylene biosynthesis, signal transduction, or metabolism. To identify such genes we have established a method for isolating ethylene mutants from a light-grown population. As a first step, we characterized the response of nutrient-starved Arabidopsis seedlings to the ethylene precursor 1-aminocyclopropane-1-carboxylic acid (ACC). We found that ACC promotes hypocotyl elongation in the light. This is in contrast to the well characterized inhibition of longitudinal expansion in the dark. Using a set of ethylene signal transduction mutants, we show that this effect of hypocotyl elongation is mediated by the same components that transduce the ethylene signal in etiolated seedlings.
MATERIALS AND METHODS
Plant Material and Growth Conditions.
The wild-type Arabidopsis thaliana (L.) Heynh. used was Columbia (Col-0; purchased from Lehle Seeds, Round Rock, TX). Ethylene response mutants, all in Col-0 background, were obtained from the Arabidopsis Biological Resource Center at Ohio State University. All mutants used in this study have been described previously (4, 6, 13–15). The ein5-1 mutant was found to be allelic to ain1 (14) and was designated ain1-10 (data not shown). For plants grown under sterile conditions, seeds were surface sterilized for 15 min in 5% sodium hypochlorite, suspended in 0.1% low-melting-point agarose, and distributed evenly on the growth medium. The growth media used were either low nutrient medium (LNM) or rich medium (MS/2). LNM consisted of 0.8% agarose in SPA Reine (Spa Monopole, Spa, Belgium) water containing 3 mg/liter Na+, 0.5 mg/liter K+, 3.5 mg/liter Ca2+, 1.3 mg/liter Mg2+, 5 mg/liter Cl−, 6.5 mg/liter SO42−, 1.9 mg/liter NO3−, 11 mg/liter HCO3−, and 6.5 mg/liter SiO2 (pH 5.8) as determined by the producer. The rich growth medium used was half-strength Murashige and Skoog (MS/2; Sigma) supplemented with 1% sucrose (pH 5.8). ACC, aminoethoxyvinylglycine (AVG), and indole-3-acetic acid (IAA) were obtained from Sigma. CoCl2 and AgNO3 were from Merck. Plates were stored at 4°C in the dark for 2 days and then put in a growth chamber at 22°C and 60% relative humidity with white fluorescent light (75 μmol/m2 per s) and long day conditions (16 h light/8 h dark). For the ethylene treatments, seeds on LNM were subjected to a continuous flow of 10 ppm ethylene for 9 days.
Measurements of Hypocotyl and Cell Length.
Measurements of hypocotyl length were performed with a Stemi SV 11 microscope (Zeiss). For the ACC and IAA treatments, hypocotyl length was determined after 2 weeks of growth. For the ethylene treatment, hypocotyls were analyzed after 9 days of exposure. Measurements of cell length were done on seedlings grown for 2 weeks on LNM, or LNM supplemented with either 50 μMACC or 50 μM ACC and 100 μM AgNO3. The tissues were cleared by incubation in chloral hydrate (3:8 ratio of water and chloral hydrate) for 24 h at room temperature and 1 h at 58°C. Cell sizes from the middle part of hypocotyls were measured using an Axioskop microscope equipped with a graduated ocular (Zeiss).
Biometric Analysis.
All results are presented as mean ± SEM. Calculations were based on at least three replicates. The statistical significance was analyzed using either Student’s t test when only two components were compared, or a one-way analysis of variance when more samples were involved.
RESULTS
Ethylene Induces Hypocotyl Elongation in Light-Grown Arabidopsis Seedlings.
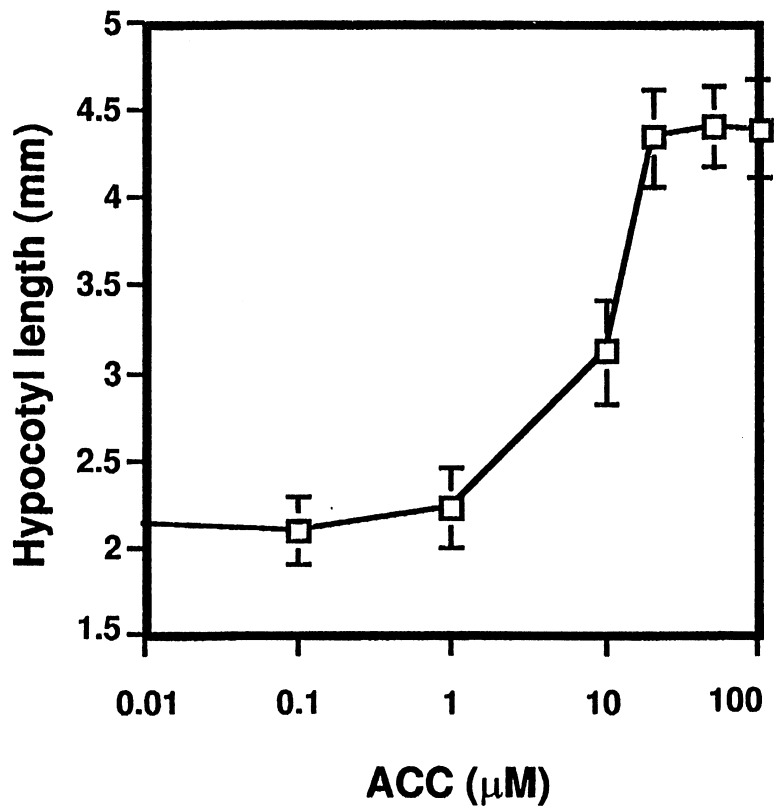
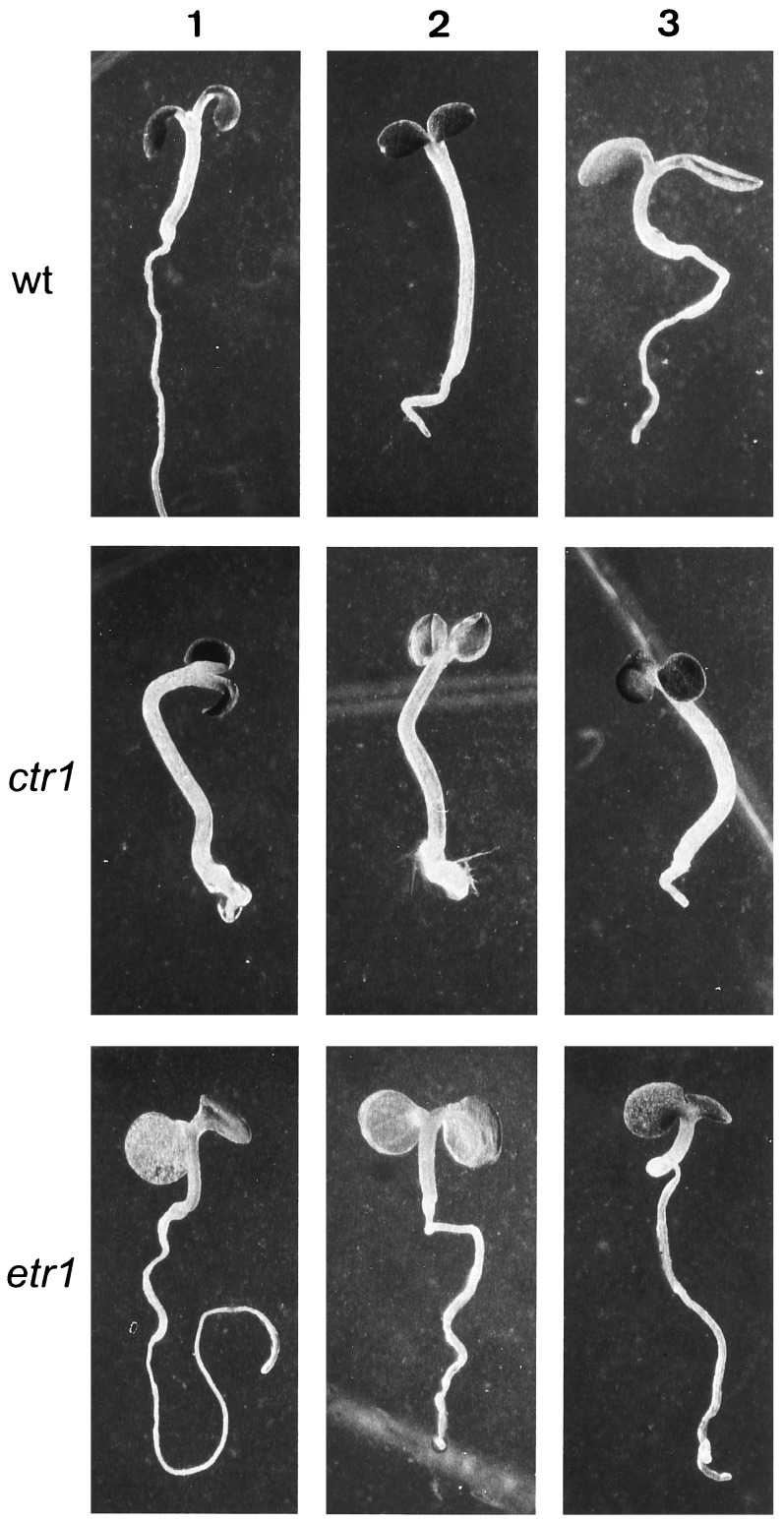
We characterized seedling responses to the ethylene precursor ACC that could potentially be useful for isolating ethylene mutants from a light-grown population. Ethylene was shown to have a dramatic effect on the size of light-grown Arabidopsis seedlings (4). However, a mutagenized population is expected to have a substantial size variability due to mutations other than those relevant for ethylene biosynthesis and action. To ensure a higher degree of uniformity of seedling size, we used a nutrient-deficient growth medium (LNM). Development on LNM led to a severe growth retardation resulting in more size uniformity. This allowed a better discrimination of mutations altering ACC responses from those affecting other biochemical or developmental pathways. While characterizing the ACC response on LNM in the light, we observed that 50 μM ACC significantly promoted hypocotyl elongation. As shown in Fig. 1, ACC induced hypocotyl growth in a dose-dependent manner. In Col-0 plants grown on LNM, a stimulation of elongation occurred at ACC concentrations above 1 μM and reached saturation at 20 μM ACC (Fig. 1). Fig. 2 illustrates the phenotypes of Col-0, ctr1-1 and etr1-3 grown on LNM supplemented with ACC or ACC plus AgNO3. The hypocotyl length of the wild type on 50 μM ACC was roughly twice the size of the untreated control. ACC stimulated hypocotyl growth during approximately 2 weeks, after which no additional effect was detected. The elongation in wild type treated with ACC could be blocked by adding 100 μM AgNO3 to the medium, suggesting an ethylene response (Fig. 2). This was supported by the fact that hypocotyl elongation could not be induced in the ethylene-insensitive mutant etr1-3, whereas the untreated ctr1-1 mutant had an elongated hypocotyl (Fig. 2).
Figure 1.
Elongation of Arabidopsis hypocotyls in the light induced by ACC. Col-0 plants were grown on LNM supplemented with ACC in a range of concentrations (0.1 μM, 1 μM, 10 μM, 20 μM, 50 μM, and 100 μM). The hypocotyl length was measured after 2 weeks of growth. Data are mean ± SEM (n = 30).
Figure 2.
Effect of ACC treatment on seedlings of Col-0 (wild type, wt), etr1-3, and ctr1-1 grown on LNM. Column 1, LNM; column 2, LNM with 50 μM ACC; column 3, LNM with 50 μM ACC and 100 μM AgNO3.
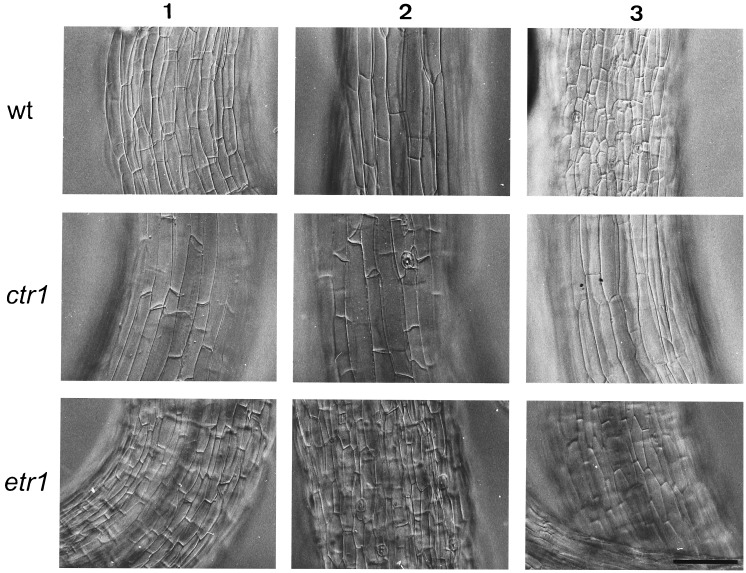
The response to ACC can be due to either an increase in cell elongation or a higher rate of cell division. To distinguish between these two possibilities, the size of epidermal hypocotyl cells was measured after ACC treatment. Elongation appeared to result from longitudinal cell expansion rather than cell division (Fig. 3; Table 1). Hypocotyl cells of ACC-treated wild-type seedlings were on average 2-fold longer than controls, correlating well with a doubling of hypocotyl length (Fig. 1; Table 1). However, on AgNO3 and in the etr1-3 mutant, hypocotyl cells were significantly shorter than in the untreated wild type, suggesting that the observed elongation was indeed an ethylene effect and that interfering with ethylene perception also blocked the action of endogenous ethylene. Epidermal cell length varied along the hypocotyl, and the data in Table 1 present cell length in the middle part. However, cells in both the apical and basal parts were still roughly 2-fold longer than comparable cells in untreated seedlings (data not shown). Final proof that the ACC-induced effects on hypocotyl length were caused by ethylene was obtained by treatment with 10 ppm of ethylene during 9 days. Ethylene fumigation led to an elongation of Col-0 hypocotyls, and this response was again absent in the etr1-3 mutant (Table 2). We analyzed the response of seedlings grown on MS/2 and found that ethylene again induced hypocotyl elongation, albeit to a much lesser extent (Table 2).
Figure 3.
Effect of ACC treatment on epidermal cells from the middle part of the hypocotyl of Col-0 (wild type, wt) and ethylene mutants ctr1-1 and etr1-3 grown on LNM. Column 1, LNM; column 2, LNM with 50 μM ACC; column 3, LNM with 50 μM ACC and 100 μM AgNO3. (Bar = 50 μm.)
Table 1.
Effect of ACC treatment on hypocotyl cell length in Col-0, ctr1-1, and etr1-3 seedlings
| Background | Treatment | Cell length, μm |
|---|---|---|
| Col-0 | Control | 112 ± 5 |
| ACC | 205 ± 10 | |
| ACC + AgNO3 | 88 ± 3 | |
| ctr1-1 | Control | 167 ± 9 |
| ACC | 173 ± 8 | |
| ACC + AgNO3 | 170 ± 8 | |
| etr1-3 | Control | 85 ± 3 |
| ACC | 83 ± 3 | |
| ACC + AgNO3 | 86 ± 3 |
Measurements of the length of epidermal cells in hypocotyls of seedlings treated with 50 μM ACC, 50 μM ACC plus 100 μM AgNO3, and controls were done after 2 weeks of growth on LNM medium in the light. The data represent the mean ± SEM of 30 measurements. All values are significantly different (P < 0.001) from Col-0 control.
Table 2.
Ethylene-induced hypocotyl elongation on media of different nutritional strength
| Background (medium) | Hypocotyl length, mm
|
|
|---|---|---|
| Air | Ethylene (10 ppm) | |
| Col-0 (LNM) | 1.05 ± 0.05 | 1.92 ± 0.01 |
| Col-0 (MS/2) | 2.37 ± 0.08 | 3.29 ± 0.08 |
| etr1-3 (LNM) | 0.96 ± 0.05 | 1.02 ± 0.05 |
| etr1-3 (MS/2) | 1.82 ± 0.08 | 1.98 ± 0.08 |
Hypocotyl length was measured after 9 days of ethylene treatment in seedlings grown on LNM and MS/2. Data for hypocotyl length are mean ± SEM of three replicates, each on 10 seedlings.
An additional effect was related to chlorophyll accumulation in elongating hypocotyls. Untreated ctr1-1 or wild-type seedlings grown on ACC consistently had white hypocotyls. In contrast, they were more green in untreated wild type. Moreover, greening was more intense in AgNO3-treated seedlings and in the strongly insensitive mutants etr1-3 and ein2-1 (data not shown).
Finally, we tested the ACC response of seedlings grown in the dark on LNM. Wild type as well as a set of ethylene mutants (see Materials and Methods) behaved similarly in a triple response assay as when grown on rich medium (i.e., MS/2) (data not shown). Thus, nutrient starvation did not affect the ethylene response in the dark.
The ACC-Induced Hypocotyl Elongation Response in Ethylene Signal Transduction Mutants.
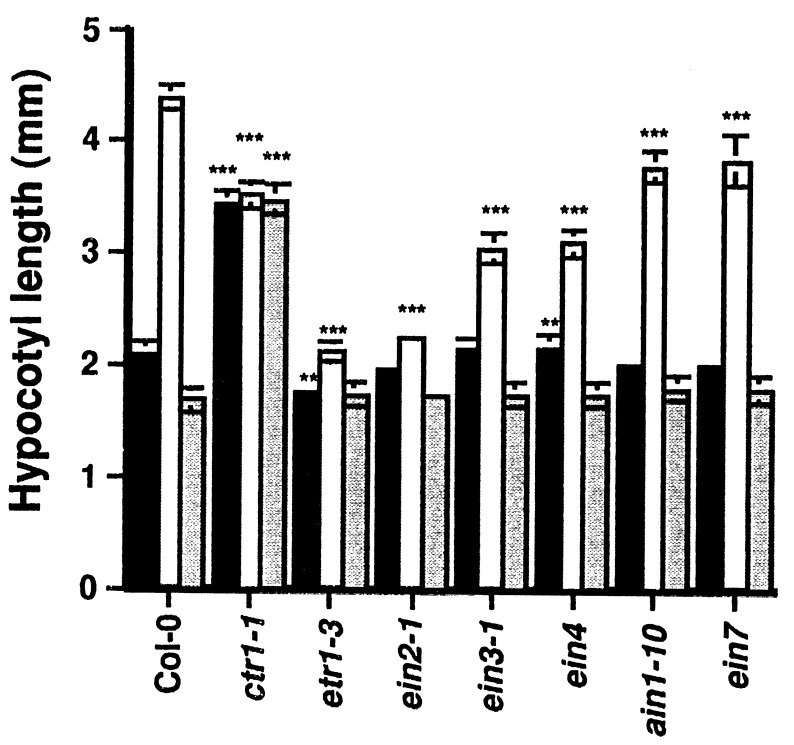
To further characterize the hypocotyl elongation response, we analyzed the hypocotyl length of a set of ethylene signal transduction mutants grown in the light on LNM containing either 50 μM ACC or 50 μM ACC and 100 μM AgNO3 (Fig. 4). On medium without ACC, the hypocotyl of the ctr1-1 mutant was ≈1.6-fold longer than wild type. This elongation was the result of cell expansion (Fig. 3). Growth on LNM supplemented with ACC or ACC plus AgNO3 did not influence the length of the ctr1-1 hypocotyls. It is important to note that the hypocotyl of ctr1-1 grown on LNM was ≈20% shorter than the ACC-treated Col-0. Elongation on LNM with ACC was inhibited in all ethylene-insensitive mutants (Fig. 4). This inhibition was most pronounced in etr1-3 and ein2-1, intermediate in ein3-1 and ein4, whereas in ain1-10 and ein7 it was weak but significant (P = 0.002 for ain1-10 and P = 0.001 for ein7). With the exception of the ein4 mutant, the strength of ACC insensitivity could be correlated with the level of ethylene insensitivity based on the triple response assay (15). The elongation of ctr1-1 on AgNO3 suggested that the observed effect on both Col-0 and the insensitive mutants was due to an inhibition of an ethylene effect and did not result from general toxicity of the AgNO3 treatment.
Figure 4.
ACC-induced hypocotyl elongation in ethylene signal transduction mutants. Col-0 and mutant strains were grown on LNM (solid bars), LNM supplemented with 50 μM ACC (open bars), and LNM containing both 50 μM ACC and 100 μM AgNO3 (grey bars). The hypocotyl lengths were measured 2 weeks after sowing. Data represent mean ± SEM (n = 30). ∗∗, P < 0.01; ∗∗∗, P < 0.001 versus Col-0 grown on the same medium.
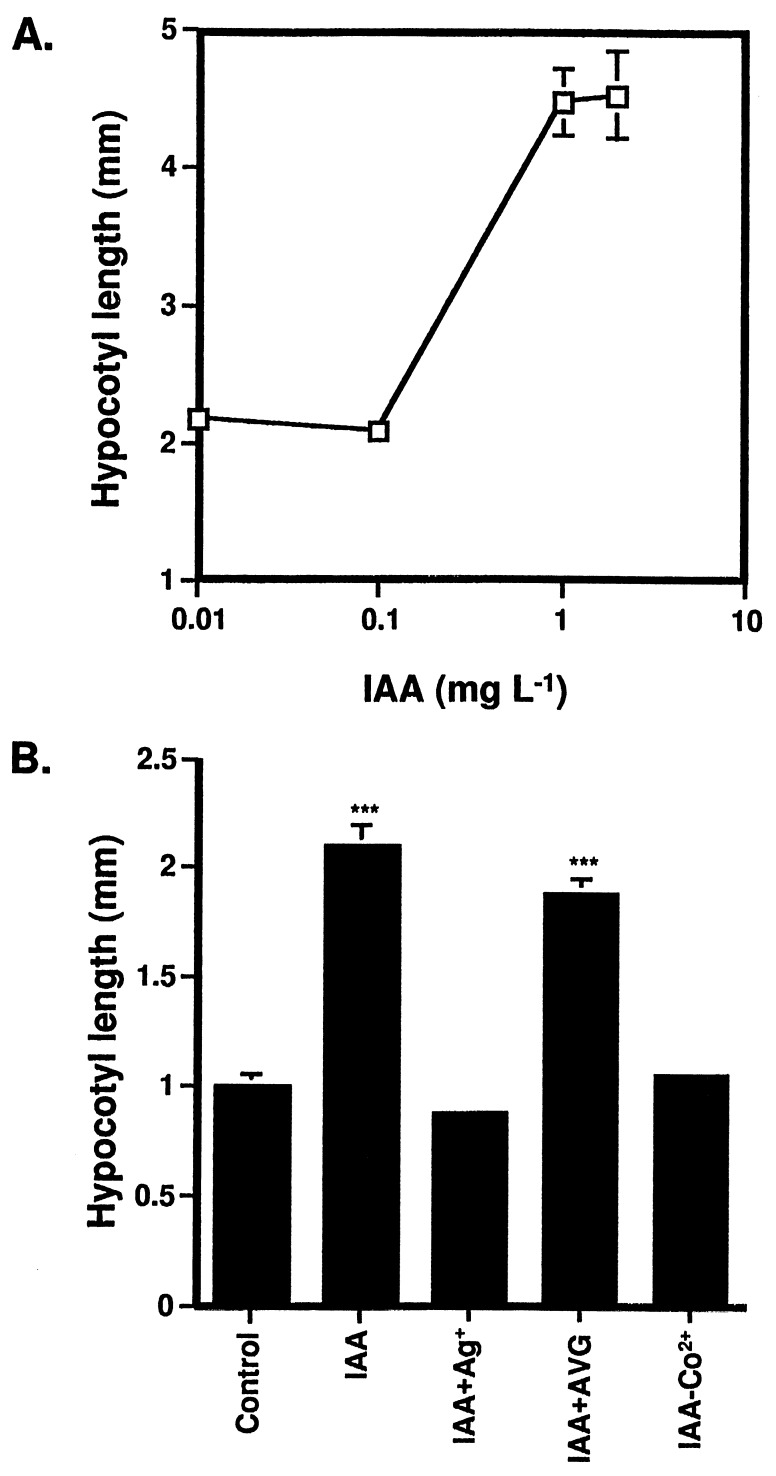
Exogenous IAA Stimulates Hypocotyl Elongation in Nutrient-Starved Seedlings.
Plants grown on LNM supplemented with IAA also showed a hypocotyl elongation in the light. In contrast, different concentrations of gibberellic acid had no effect (data not shown). The dose-response curve (Fig. 5A) showed that 1 mg/liter IAA (6 μM) induced an elongation of ≈2-fold, which was primarily the result of longitudinal cell expansion (data not shown). A higher IAA level did not lead to further elongation (Fig. 5A). Because the ACC and IAA responses are similar in strength and IAA is a known inducer of ethylene biosynthesis (16), we tested whether the IAA response was mediated by ethylene. The ethylene action inhibitor Ag+ completely blocked the IAA effect (Fig. 5B). However, application of inhibitors of ethylene biosynthesis resulted in conflicting data. Growth on LNM containing 200 μM CoCl2 strongly inhibited the IAA response. Whereas 2 μM AVG had no effect, 10 μM AVG resulted in bleaching of the cotyledons, but still did not interfere with the IAA-induced elongation (data not shown). On MS/2 medium, 1 mg/liter IAA could not stimulate elongation (data not shown).
Figure 5.
IAA-induced hypocotyl elongation. The hypocotyl length was measured after 2 weeks of growth in the light. Data are mean ± SEM (n = 30). (A) Col-0 plants grown on LNM supplemented with a range of IAA concentrations (0.01 mg/liter, 0.1 mg/liter, 1 mg/liter, 2 mg/liter). (B) Effects of ethylene biosynthesis and action inhibitors on auxin-induced hypocotyl elongation (IAA, 1 mg/liter; Ag+, 100 μM; AVG, 2 μM; Co2+, 200 μM). ∗∗∗, P < 0.001 versus Col-0 grown on LNM.
DISCUSSION
Ethylene has been shown to inhibit hypocotyl elongation in etiolated pea seedlings (17). This response was observed in numerous plant species and has been used in isolating a number of Arabidopsis ethylene mutants (12). In this study we show that ethylene or its precursor ACC have an opposite effect on hypocotyl elongation in Arabidopsis seedlings grown in the light. This effect is most pronounced in seedlings grown on nutrient-deficient medium (≈2-fold elongation) and is detectable to a lesser extent in seedlings grown on MS/2 medium (≈30% elongation). In the dark, seedlings on nutrient-deficient medium display normal ethylene responses (i.e., inhibition of hypocotyl elongation, radial expansion, and exaggerated apical hook curvature). The ethylene-induced elongation in the light observed in this study appears to be the result of an increase in cell elongation rather than division. Several of the mutations causing ethylene insensitivity in etiolated seedlings also result in an inhibition of the effect of ethylene in the light. In fact, the relative strength of ethylene insensitivity based on the hypocotyl elongation response in the light is similar to the level of insensitivity as determined by the triple response (15). This suggests that in inhibiting hypocotyl elongation in the dark as well as in promoting elongation in the light, the ethylene signal is likely to be transduced by the same pathway. The opposite effects in light and dark could suggest the involvement of light-regulated upstream or downstream components. Interestingly, the ein4 mutant had a weaker relative ethylene insensitivity than expected from its response in etiolated seedlings. The position of the EIN4 gene in the signal transduction pathway is thought to be upstream of CTR1 (15). It is not yet clear whether EIN4 acts downstream or in parallel to ETR1 as one of the putative ethylene receptors (18).
Exogenous auxin also promotes hypocotyl elongation in nutrient-deficient seedlings, an induction that was not visible in seedlings grown on MS/2 medium. The ACC and IAA effects appear to be similar, with a response that becomes saturated at ≈2-fold elongation. Moreover, both treatments inhibit the expansion of cotyledons, which in each case seems to be caused by a reduction of cell enlargement (J.S., unpublished results). Previously, it has been shown that raising endogenous auxin levels in transgenic Arabidopsis results in a longer hypocotyl (19). However, no elongation was found upon exogenous auxin application in Arabidopsis. Apparently, the effects of ACC and IAA observed on LNM are partially or totally masked on nutrient-rich growth medium. This might be due to the presence of optimal endogenous concentrations. A number of interpretations are possible. First, in seedlings grown on nutrient-deficient medium a direct inhibition of cotyledon cell expansion by ACC (together with an inhibition of root growth) might result in a significant increase of nutrients available for hypocotyl growth. Indeed, hypocotyl cells of seedlings grown on LNM containing ACC are not only longer, but are also often wider than in untreated hypocotyls, resulting in a large increase in cell volume. This would imply that different cells in an Arabidopsis seedling display different levels of ethylene sensitivity. In this case, ethylene would function as a regulator of sink to source relations, favoring growth of the hypocotyl (i.e., organs and cells with lower ethylene sensitivity) by inhibiting cotyledon expansion and root growth. Alternatively, ethylene (or ACC) and auxins might directly promote expansion of hypocotyl cells thus limiting growth of cotyledons and roots on a nutrient-deficient medium. Finally, a rich nutrient medium might allow the uptake or synthesis of inhibitors of ACC and IAA elongation responses, inhibitors that would not be available on LNM.
As auxins are known inducers of ethylene biosynthesis (10) and as the ACC and IAA responses are similar, we tested whether IAA-induced hypocotyl elongation is mediated by ethylene. Although Ag+ inhibited the IAA response, treatments with the ethylene biosynthesis inhibitors AVG and CoCl2—blocking the ACC synthase and ACC oxidase activities, respectively—yielded conflicting data. Results from experiments using such inhibitors should be interpreted with caution (20, 21). Clearly, further studies are required to determine the role of ethylene in the IAA-induced elongation. It should be tested whether AVG and CoCl2 indeed inhibit ethylene production in seedlings on LNM. Although its existence remains to be proved, an AVG-insensitive ACC synthase isoform might be involved. Alternatively, CoCl2 might directly inhibit hypocotyl elongation without interfering with ethylene biosynthesis. It should be analyzed whether the IAA response is affected in ethylene-insensitive mutant backgrounds. Initial experiments suggest that this is the case, although in the etr1-3 mutant the IAA response appeared to be retarded but not absent (J.S., unpublished results). There are several reports on the cross-talk between ethylene and auxin pathways, also in Arabidopsis. Most of the auxin-resistant mutants proved to be ethylene insensitive as well (8). An ACC synthase gene was cloned that is primary IAA responsive (22). The HLS1 gene was shown to mediate ethylene effects by regulating auxin activity (7). Interestingly, hls1 mutants display a reduction in hypocotyl and root length in combination with elongated cotyledons.
Ethylene can promote elongation of tissues in a range of aquatic and semiaquatic plants including rice coleoptiles and stems (23, 24) as well as petioles and flower stalks of Ranunculus sceleratus (25, 26). Furthermore, it can induce growth of petals and styles in immature carnation flowers (27) and of anther filaments in Brussels sprouts (28). To date, it is not clear whether these various elongation effects are resulting from similar mechanisms or signaling pathways. The ACC- and IAA-induced hypocotyl elongation reported in this study provides a simple assay for studying auxin and ethylene responses in the light. We used these ACC responses in nutrient-starved seedlings to establish a screen for isolating mutants from light-grown populations. Screening nutrient-starved seedlings has several advantages. Space requirements are reduced to a minimum, and because growth and development are retarded and are largely based on nutrients present in the cotyledons, more size uniformity is obtained. In addition to the hypocotyl elongation response, we have observed other ACC responses that are not seen in seedlings grown on Murashige and Skoog medium (J.S., unpublished results). Scoring for hypocotyl length is also more practical in nutrient-starved seedlings. As leaf development is severely retarded, hypocotyls can be observed throughout a longer time interval. Molecular-genetic characterization of mutants from this screen may reveal new insights into the role of ethylene in vegetative growth and unravel novel components of the ethylene signaling pathway.
Acknowledgments
We thank the Ohio Stock Center for providing the ethylene mutants, Mike May for critical reading of the manuscript, Wim Van Caeneghem for technical assistance, Martine De Cock for help in preparing the manuscript, and Karel Spruyt and Rebecca Verbanck for figures and photographs. This work was supported by a grant from the Vlaams Actieprogramma Biotechnologie (ETC 002). D.V.D.S. is a Research Associate of the Fund for Scientific Research–Flanders (Belgium).
ABBREVIATIONS
- ACC
1-aminocyclopropane-1-carboxylic acid
- AVG
aminoethoxyvinylglycine
- IAA
indole-3-acetic acid
- LNM
low nutrient medium
- MS/2
half-strength Murashige and Skoog medium
References
- 1.Cosgrove D. Annu Rev Plant Physiol Plant Mol Biol. 1986;37:377–405. doi: 10.1146/annurev.pp.37.060186.002113. [DOI] [PubMed] [Google Scholar]
- 2.Shibaoka H. Annu Rev Plant Physiol Plant Mol Biol. 1994;45:527–544. [Google Scholar]
- 3.Abeles F B. In: Ethylene in Plant Biology. 2nd Ed. Abeles F B, Morgan P W, Salveit M E Jr, editors. New York: Academic; 1992. pp. 145–156. [Google Scholar]
- 4.Kieber J J, Rothenberg M, Roman G, Feldmann K A, Ecker J R. Cell. 1993;72:427–441. doi: 10.1016/0092-8674(93)90119-b. [DOI] [PubMed] [Google Scholar]
- 5.Rodrigues-Pousada R A, De Rycke R, Dedonder A, Van Caeneghem W, Engler G, Van Montagu M, Van Der Straeten D. Plant Cell. 1993;5:897–911. doi: 10.1105/tpc.5.8.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bleecker A B, Estelle M A, Somerville C, Kende H. Science. 1988;241:1086–1089. doi: 10.1126/science.241.4869.1086. [DOI] [PubMed] [Google Scholar]
- 7.Lehman A, Black R, Ecker J R. Cell. 1996;85:183–194. doi: 10.1016/s0092-8674(00)81095-8. [DOI] [PubMed] [Google Scholar]
- 8.Hobbie L, Estelle M. Plant Cell Environ. 1994;17:525–540. doi: 10.1111/j.1365-3040.1994.tb00147.x. [DOI] [PubMed] [Google Scholar]
- 9.Pickett F B, Wilson A K, Estelle M. Plant Physiol. 1990;94:1462–1466. doi: 10.1104/pp.94.3.1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang S F, Hoffman N E. Annu Rev Plant Physiol. 1984;35:155–189. [Google Scholar]
- 11.Osborne D J. In: Biochemical and Physiological Aspects of Ethylene Production in Lower and Higher Plants, Advances in Agricultural Biotechnology. Clijsters H, De Proft M, Marcelle R, Van Poucke M, editors. Vol. 26. Dordrecht, The Netherlands: Kluwer; 1989. pp. 1–11. [Google Scholar]
- 12.Ecker J R. Science. 1995;268:667–675. doi: 10.1126/science.7732375. [DOI] [PubMed] [Google Scholar]
- 13.Guzmán P, Ecker J R. Plant Cell. 1990;2:513–523. doi: 10.1105/tpc.2.6.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Van Der Straeten D, Djudzman A, Van Caeneghem W, Smalle J, Van Montagu M. Plant Physiol. 1993;102:401–408. doi: 10.1104/pp.102.2.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roman G, Lubarsky B, Kieber J J, Rothenberg M, Ecker J R. Genetics. 1995;139:1393–1409. doi: 10.1093/genetics/139.3.1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McKeon T A, Yang S-F. In: Plant Hormones and Their Role in Plant Growth and Development. Davies P J, editor. Dordrecht, The Netherlands: Nijhoff; 1987. pp. 94–112. [Google Scholar]
- 17.Neljubow D. Beih Bot Zentralbl. 1901;10:128–139. [Google Scholar]
- 18.Chang C. Trends Biochem Sci. 1996;21:129–133. [PubMed] [Google Scholar]
- 19.Romano C P, Robson P H, Smith H, Estelle M, Klee H. Plant Mol Biol. 1995;27:1071–1083. doi: 10.1007/BF00020881. [DOI] [PubMed] [Google Scholar]
- 20.Biddington N L. Plant Growth Regul. 1992;11:173–187. [Google Scholar]
- 21.Nissen P. Physiol Plant. 1994;92:397–403. [Google Scholar]
- 22.Abel S, Nguyen M D, Chow W, Theologis A. J Biol Chem. 1995;270:19093–19099. doi: 10.1074/jbc.270.32.19093. [DOI] [PubMed] [Google Scholar]
- 23.Ku H S, Suge H, Rappaport L, Pratt H K. Planta. 1970;90:333–339. doi: 10.1007/BF00386385. [DOI] [PubMed] [Google Scholar]
- 24.Métraux J-P, Kende H. Plant Physiol. 1983;72:441–446. doi: 10.1104/pp.72.2.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Musgrave A, Walters J. New Phytol. 1973;72:783–789. [Google Scholar]
- 26.Samarakoon A B, Horton R F. Can J Bot. 1981;59:1386–1392. [Google Scholar]
- 27.Camprubi P, Nichols R. J Hortic Sci. 1979;54:225–228. [Google Scholar]
- 28.Biddington N L, Robinson H T. Plant Growth Regul. 1993;12:29–35. [Google Scholar]