Abstract
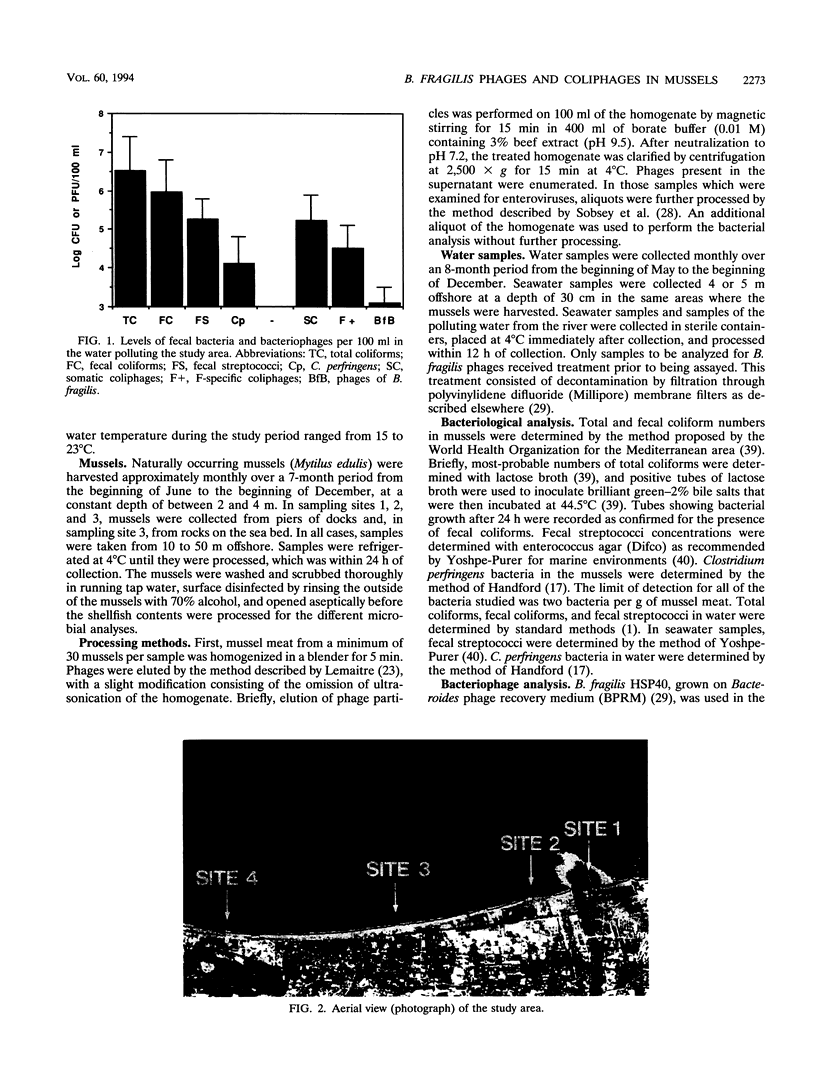
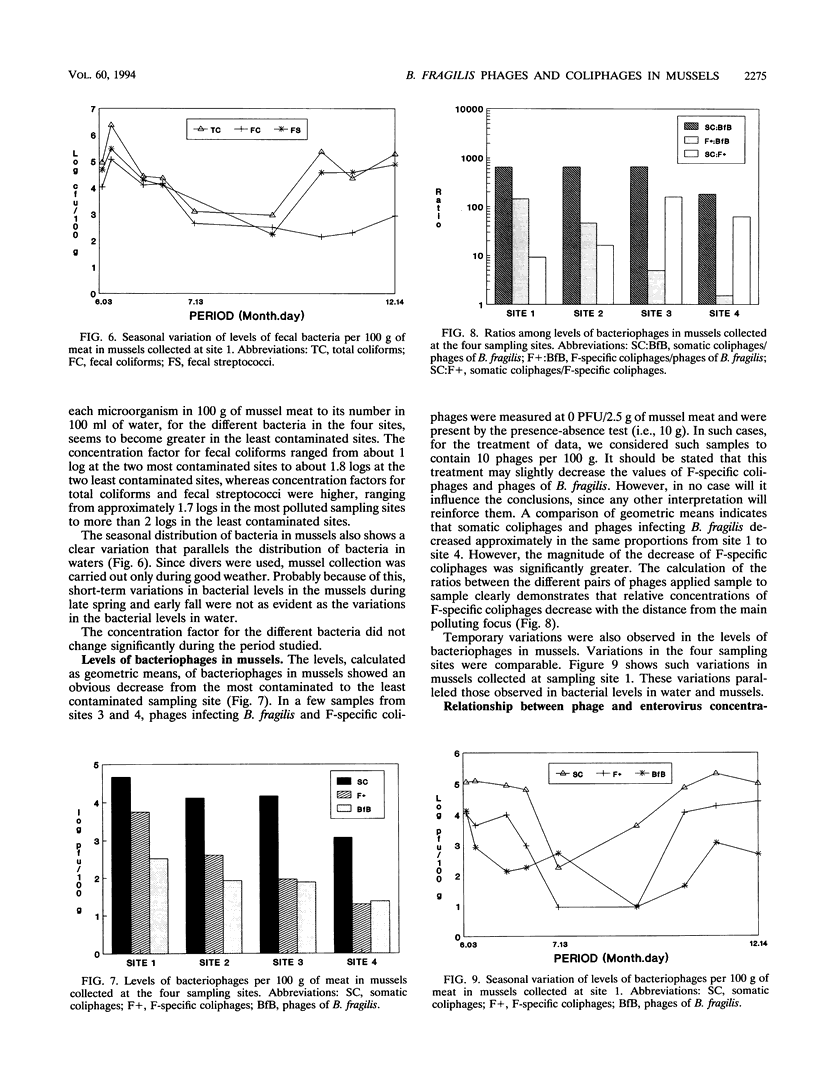
Concentrations of fecal bacteria, somatic and F-specific coliphages, and phages infecting Bacteroides fragilis in naturally occurring black mussels (Mytilus edulis) were determined. Mussels were collected over a 7-month period at four sampling sites with different levels of fecal pollution. Concentrations of both fecal bacteria and bacteriophages in mussel meat paralleled the concentration of fecal bacteria in the overlying waters. Mussels bioaccumulated efficiently, although with different efficiencies, all of the microorganisms studied. Ratios comparing the levels of microorganisms in mussels were determined. These ratios changed in mussels collected at the different sites. They suggest that bacteriophages infecting B. fragilis and somatic coliphages have the lowest decay rates among the microorganisms studied, with the exception of Clostridium perfringens. On the contrary, concentrations of F-specific coliphages showed a greater rate of decay than the other bacteriophages at sites more distant from the focus of contamination. Additionally, levels of enteroviruses were studied in a number of samples, and in these samples, the B. fragilis bacteriophages clearly outnumbered the enteroviruses. The results of this study indicate that, under the environmental conditions studied, the fate of phages infecting B. fragilis released into the marine environment resembles that of human viruses more than any other microorganism examined.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armon R., Arella M., Payment P. A highly efficient second-step concentration technique for bacteriophages and enteric viruses using ammonium sulfate and Tween 80. Can J Microbiol. 1988 May;34(5):651–655. doi: 10.1139/m88-107. [DOI] [PubMed] [Google Scholar]
- Canzonier W. J. Accumulation and elimination of coliphage S-13 by the hard clam, Mercenaria mercenaria. Appl Microbiol. 1971 Jun;21(6):1024–1031. doi: 10.1128/am.21.6.1024-1031.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debartolomeis J., Cabelli V. J. Evaluation of an Escherichia coli host strain for enumeration of F male-specific bacteriophages. Appl Environ Microbiol. 1991 May;57(5):1301–1305. doi: 10.1128/aem.57.5.1301-1305.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desenclos J. C., Klontz K. C., Wilder M. H., Nainan O. V., Margolis H. S., Gunn R. A. A multistate outbreak of hepatitis A caused by the consumption of raw oysters. Am J Public Health. 1991 Oct;81(10):1268–1272. doi: 10.2105/ajph.81.10.1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerba C. P., Goyal S. M., LaBelle R. L., Cech I., Bodgan G. F. Failure of indicator bacteria to reflect the occurrence of enteroviruses in marine waters. Am J Public Health. 1979 Nov;69(11):1116–1119. doi: 10.2105/ajph.69.11.1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goyal S. M., Gerba C. P., Melnick J. L. Human enteroviruses in oysters and their overlying waters. Appl Environ Microbiol. 1979 Mar;37(3):572–581. doi: 10.1128/aem.37.3.572-581.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handford P. M. A new medium for the detection and enumeration of Clostridium perfringens in foods. J Appl Bacteriol. 1974 Dec;37(4):559–570. doi: 10.1111/j.1365-2672.1974.tb00481.x. [DOI] [PubMed] [Google Scholar]
- Havelaar A. H., Hogeboom W. M. A method for the enumeration of male-specific bacteriophages in sewage. J Appl Bacteriol. 1984 Jun;56(3):439–447. doi: 10.1111/j.1365-2672.1984.tb01372.x. [DOI] [PubMed] [Google Scholar]
- Portnoy B. L., Mackowiak P. A., Caraway C. T., Walker J. A., McKinley T. W., Klein C. A., Jr Oyster-associated hepatitis. Failure of shellfish certification programs to prevent outbreaks. JAMA. 1975 Sep 8;233(10):1065–1068. doi: 10.1001/jama.233.10.1065. [DOI] [PubMed] [Google Scholar]
- Sobsey M. D., Carrick R. J., Jensen H. R. Improved methods for detecting enteric viruses in oysters. Appl Environ Microbiol. 1978 Jul;36(1):121–128. doi: 10.1128/aem.36.1.121-128.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tartera C., Araujo R., Michel T., Jofre J. Culture and decontamination methods affecting enumeration of phages infecting Bacteroides fragilis in sewage. Appl Environ Microbiol. 1992 Aug;58(8):2670–2673. doi: 10.1128/aem.58.8.2670-2673.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tartera C., Jofre J. Bacteriophages active against Bacteroides fragilis in sewage-polluted waters. Appl Environ Microbiol. 1987 Jul;53(7):1632–1637. doi: 10.1128/aem.53.7.1632-1637.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tartera C., Lucena F., Jofre J. Human origin of Bacteroides fragilis bacteriophages present in the environment. Appl Environ Microbiol. 1989 Oct;55(10):2696–2701. doi: 10.1128/aem.55.10.2696-2701.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truman B. I., Madore H. P., Menegus M. A., Nitzkin J. L., Dolin R. Snow Mountain agent gastroenteritis from clams. Am J Epidemiol. 1987 Sep;126(3):516–525. doi: 10.1093/oxfordjournals.aje.a114684. [DOI] [PubMed] [Google Scholar]
- Yoshpe-Purer Y. Evaluation of media for monitoring fecal streptococci in seawater. Appl Environ Microbiol. 1989 Aug;55(8):2041–2045. doi: 10.1128/aem.55.8.2041-2045.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]




