Abstract
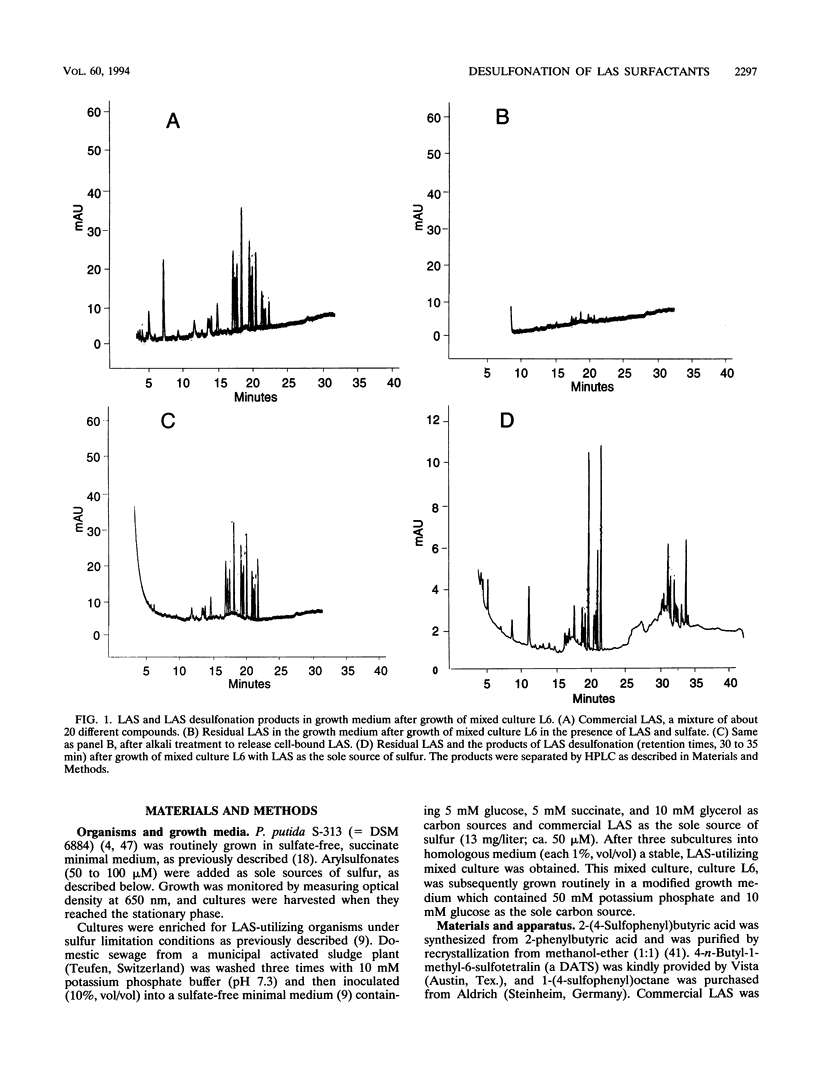
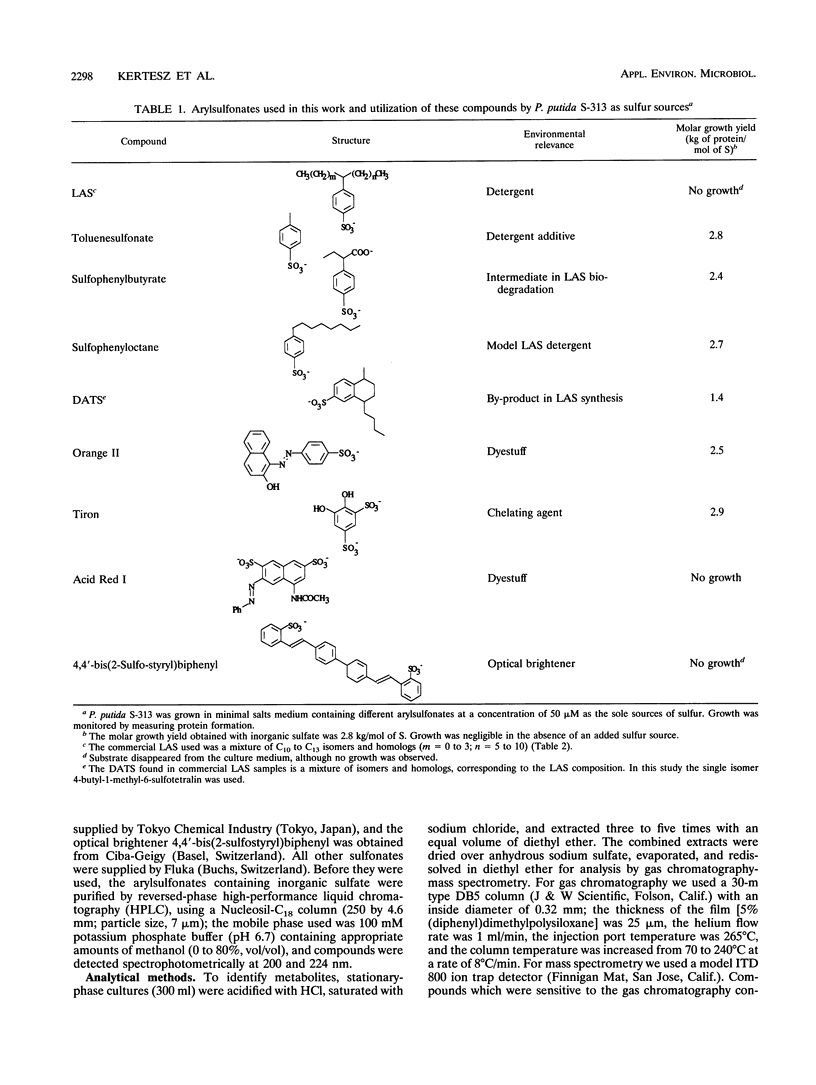
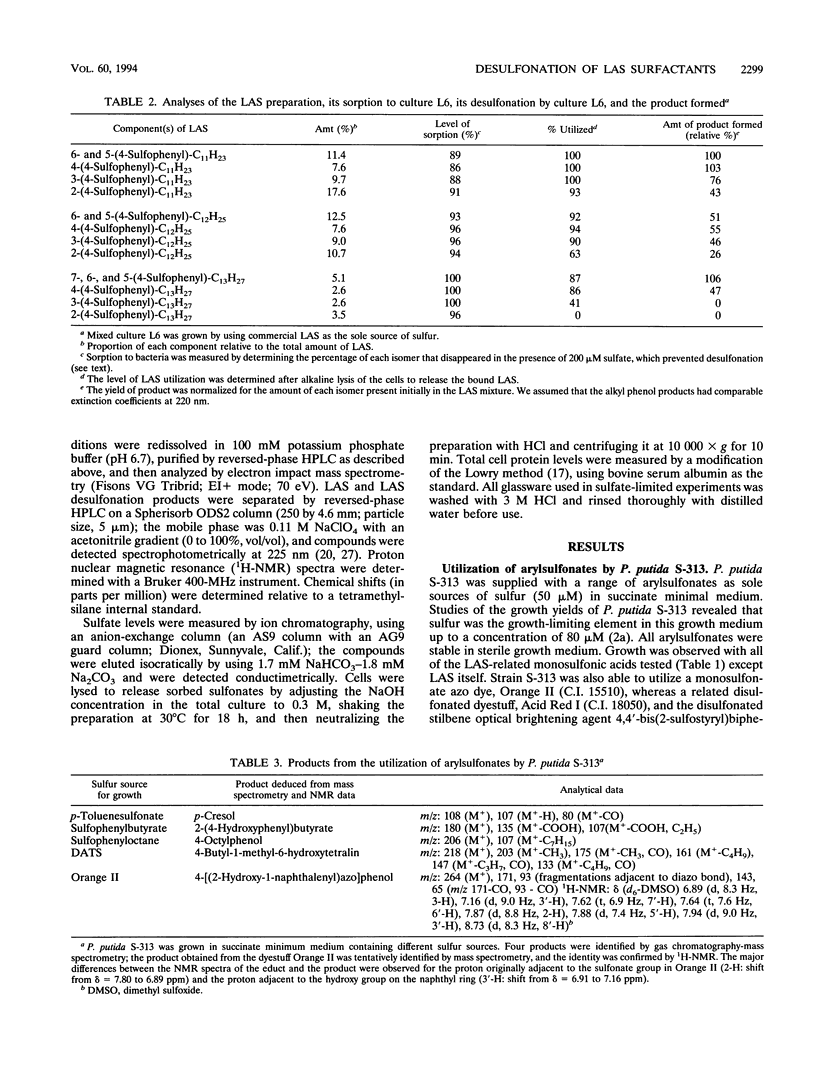
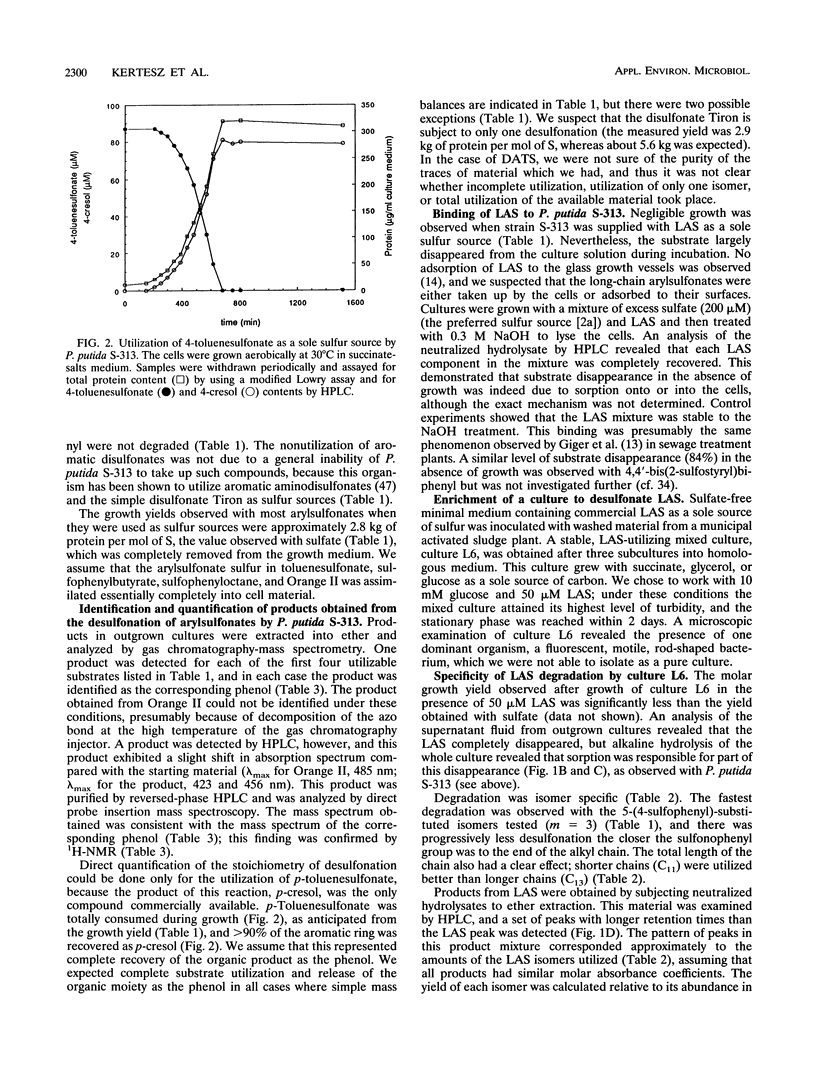
Pseudomonas putida S-313 (= DSM 6884) grew in sulfate-free medium when the sole sulfur source supplied was one of several arylsulfonates involved in the synthesis, application, or biodegradation of linear alkyl-benzenesulfonate (LAS) surfactants. 2-(4-Sulfophenyl)butyric acid, 4-n-butyl-1-methyl-6-sulfotetralin, and 4-toluenesulfonic acid were each completely utilized during growth, as were the model LAS 1-(4-sulfophenyl) octane and the arylsulfonate dyestuff Orange II. The product in each case was the corresponding phenol, which was identified by gas chromatography-mass spectrometry or 1H nuclear magnetic resonance. Stoichiometric conversion of 4-toluenesulfonic acid to 4-cresol was observed. The molar growth yields observed were 2.4 to 2.8 kg of protein per mol of S, which were comparable to the yield for sulfate. Commercial LAS disappeared from growth medium inoculated with strain S-313, but negligible growth occurred; digestion of cells in alkali led to recovery of the LAS mixture, which seemingly sorbed to the cells. However, mixed culture L6 was readily obtained from batch enrichment cultures containing commercial LAS as a sole sulfur source and an inoculum from domestic sewage. Culture L6 desulfonated components of the LAS surfactant to the corresponding phenols, which were identified by gas chromatography-mass spectrometry. Compounds with shorter alkyl chains were desulfonated preferentially, as were the centrally substituted isomers. In the presence of 200 μM sulfate, culture L6 grew well and LAS disappeared, although this was due purely to sorption, as shown by digestion of the cells in alkali. Thus, under sulfate-limited conditions, LAS can be desulfonated directly.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anliker R. Color chemistry and the environment. Ecotoxicol Environ Saf. 1977 Sep;1(2):211–237. doi: 10.1016/0147-6513(77)90037-9. [DOI] [PubMed] [Google Scholar]
- Busse H., El-Banna T., Auling G. Evaluation of different approaches for identification of xenobiotic- degrading pseudomonads. Appl Environ Microbiol. 1989 Jun;55(6):1578–1583. doi: 10.1128/aem.55.6.1578-1583.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung K. T., Stevens S. E., Jr, Cerniglia C. E. The reduction of azo dyes by the intestinal microflora. Crit Rev Microbiol. 1992;18(3):175–190. doi: 10.3109/10408419209114557. [DOI] [PubMed] [Google Scholar]
- Cook A. M., Hütter R. Ametryne and prometryne as sulfur sources for bacteria. Appl Environ Microbiol. 1982 Apr;43(4):781–786. doi: 10.1128/aem.43.4.781-786.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feigel B. J., Knackmuss H. J. Syntrophic interactions during degradation of 4-aminobenzenesulfonic acid by a two species bacterial culture. Arch Microbiol. 1993;159(2):124–130. doi: 10.1007/BF00250271. [DOI] [PubMed] [Google Scholar]
- Haug W., Schmidt A., Nörtemann B., Hempel D. C., Stolz A., Knackmuss H. J. Mineralization of the sulfonated azo dye Mordant Yellow 3 by a 6-aminonaphthalene-2-sulfonate-degrading bacterial consortium. Appl Environ Microbiol. 1991 Nov;57(11):3144–3149. doi: 10.1128/aem.57.11.3144-3149.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiménez L., Breen A., Thomas N., Federle T. W., Sayler G. S. Mineralization of linear alkylbenzene sulfonate by a four-member aerobic bacterial consortium. Appl Environ Microbiol. 1991 May;57(5):1566–1569. doi: 10.1128/aem.57.5.1566-1569.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy S. I., Fewson C. A. Enzymes of the mandelate pathway in Bacterium N.C.I.B. 8250. Biochem J. 1968 Apr;107(4):497–506. doi: 10.1042/bj1070497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kertesz M. A., Leisinger T., Cook A. M. Proteins induced by sulfate limitation in Escherichia coli, Pseudomonas putida, or Staphylococcus aureus. J Bacteriol. 1993 Feb;175(4):1187–1190. doi: 10.1128/jb.175.4.1187-1190.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locher H. H., Leisinger T., Cook A. M. 4-Sulphobenzoate 3,4-dioxygenase. Purification and properties of a desulphonative two-component enzyme system from Comamonas testosteroni T-2. Biochem J. 1991 Mar 15;274(Pt 3):833–842. doi: 10.1042/bj2740833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locher H. H., Thurnheer T., Leisinger T., Cook A. M. 3-nitrobenzenesulfonate, 3-aminobenzenesulfonate, and 4-aminobenzenesulfonate as sole carbon sources for bacteria. Appl Environ Microbiol. 1989 Feb;55(2):492–494. doi: 10.1128/aem.55.2.492-494.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paszczynski A., Pasti-Grigsby M. B., Goszczynski S., Crawford R. L., Crawford D. L. Mineralization of sulfonated azo dyes and sulfanilic acid by Phanerochaete chrysosporium and Streptomyces chromofuscus. Appl Environ Microbiol. 1992 Nov;58(11):3598–3604. doi: 10.1128/aem.58.11.3598-3604.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigoillot J. C., Nguyen M. H. Complete oxidation of linear alkylbenzene sulfonate by bacterial communities selected from coastal seawater. Appl Environ Microbiol. 1992 Apr;58(4):1308–1312. doi: 10.1128/aem.58.4.1308-1312.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willetts A. J., Cain R. B. Microbial metabolism of alkylbenzene sulphonates. Bacterial metabolism of undecylbenzene-p-sulphonate and dodecylbenzene-p-sulphonate. Biochem J. 1972 Sep;129(2):389–402. doi: 10.1042/bj1290389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann T., Kulla H. G., Leisinger T. Properties of purified Orange II azoreductase, the enzyme initiating azo dye degradation by Pseudomonas KF46. Eur J Biochem. 1982 Dec;129(1):197–203. doi: 10.1111/j.1432-1033.1982.tb07040.x. [DOI] [PubMed] [Google Scholar]
- Zürrer D., Cook A. M., Leisinger T. Microbial desulfonation of substituted naphthalenesulfonic acids and benzenesulfonic acids. Appl Environ Microbiol. 1987 Jul;53(7):1459–1463. doi: 10.1128/aem.53.7.1459-1463.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]



