Abstract
Ambiguous spatial behavior deficits induced in adult rats by different types of dentate gyrus lesions were examined by subjecting neonatal rats to x-ray irradiation, which reduces the granule cell population in fascia dentata without affecting the number of hilar neurons and pyramidal cells of Ammon’s horn. Three- to six-month-old irradiated and intact male Long–Evans rats were tested in the Morris water maze. Four experiments were done. (i) Rats were trained to find an invisible escape platform, when started from any of four equidistant points at the circumference of the pool. (ii) The same rats then were trained to find a visible platform in the same pool. Poor performance of irradiated rats in both experiments suggested a visual deficit. (iii) Navigation in the absence of visual cues was studied in other rats trained in total darkness to find the escape platform under conditions of fixed start–fixed goal geometry. (iv) Contribution of nonvisual allocentric cues and egocentric path integration mechanisms to spatial performance of the above rats was tested in darkness after rotating both the start and goal positions by 90° clockwise. Impairment of irradiated rats in Exp. 3 and 4 and histological examination of their brains support the conclusion that 60–70% reduction of granule cells in the dorsal hippocampus causes significant deterioration in both allocentric and egocentric orientation.
Keywords: spatial memory, allocentric orientation, path integration, dentate gyrus, granule cells
There is considerable evidence indicating that the rat hippocampal formation is critically involved in the neural representation of spatial navigation (1, 2). Spatial localization is seriously impaired after different kinds of hippocampal damage (3). Pyramidal cells in the hippocampus discharge selectively at specific locations of the environment (4, 5). Notwithstanding this information about the effects of global hippocampal lesions on spatial learning, the contribution of discrete subregions of the hippocampus is less well understood (3, 6, 7). Studies using the Morris water maze have revealed important differences between selective lesions of the dorsal and ventral hippocampus (6), and the dorsal CA1 and ventral CA3 subregions (7). Data about the contribution of the dentate gyrus, which is the first stage of the intrahippocampal trisynaptic loop, are still contradictory (3, 8, 9). Colchicine-induced lesions of this structure caused severe spatial learning deficit (3, 9), whereas selective destruction of as much as 80% of the granule cells by adrenalectomy was almost ineffective (8). It should be noted that colchicine-induced lesion always involves a severe destruction of the hilar region and the CA3 subregion of Ammon’s horn.
Unlike differentiating and mature cells of the nervous system, multiplying cells are extremely radiosensitive (10). This allows selective elimination of the postnatally forming granule cells of the dentate gyrus (11, 12). Fractionated x-ray irradiation of specific brain regions that produce selective and massive reduction in the number of granule cells caused several behavioral signs of hippocampal damage, e.g., perseveration in T-maze exploration, retarded acquisition of passive avoidance, and increased horizontal locomotion (12, 13). The effects of neonatal x-irradiation are substantially different from those elicited by colchicine lesions or by adrenalectomy in adult rats, because the former prevents formation of granule cells and related circuits, whereas the latter destroys already-established structures.
Does neonatal x-ray irradiation lead to serious behavioral deficits in adulthood, or is the early damage easily compensated during development? What magnitude of neonatal insult could be compensated for without detectable behavioral change? What magnitude of neonatal insult leads to typical behavioral impairments and causes changes of place cell activity (9)?
The advantage of the single high dose of x-irradiation is that with adequate timing of the irradiation during the first 3 weeks of postnatal life it is possible to control the number of granule cells that are allowed to develop, and thus to determine the granule cell loss leading to typical behavioral deficits. In the present study, the highest possible dose of x-ray irradiation was applied on the first postnatal day to elicit a large loss of granule cells and a maximum navigation impairment.
MATERIALS AND METHODS
Animals.
Long–Evans hooded rats obtained from the Charles River Breeding Laboratories were used for breeding the animals used in this study. The rats were housed in the University Medical School of Pécs, where the parent animals were kept under standard conditions with light cycle of 12-h dark, 12-h light and free access to lab chow and water.
Pregnant females were isolated. Six to 18 h after birth, pups were taken for the x-ray irradiation and were returned immediately afterwards to their mothers. Loss of the hair on the head that could be determined between the 10th to 20th postnatal days indicated the location and effectiveness of the irradiation. Improperly irradiated rats were removed from the litter. The animals were weaned after the 40th postnatal day, and experimental and control groups were formed by mixing animals from three to five litters. Three- to five-month-old male animals were used, but the experimental and control groups in each experiment included animals of the same age.
X-Ray Irradiation.
Litters were divided between the x-ray irradiated or nonirradiated (control) groups. Pups, under light ether anesthesia, were slipped into a lead-coated tube, which covered the whole body and the lower part of the head, including the cerebellum. X-rays (therapeutical x-ray equipment, 60 kV) were delivered dorsoventrally. Two to four pups received x-ray irradiation (approximately 180 rad/min for 5 min) at the same time. This way rats received a total dose of 800-900 rads. In Exp. 1 and 2, the cerebellum was shielded, but the eyes were not. In Exp. 3, a lead cover was used with a circle of 5 mm in diameter over the middle of the cranium that corresponded the location of the dorsal hippocampi. The shielding was possible, because this equipment emitted an x-ray of low penetration rate with a 50% absorption in 1.5-cm layer of skin-equivalent tissue. According the control measurements a 1-mm thick lead plate effectively reduced the x-ray dose to 0 rad. It has to be emphasized that in all of the cases only the heads of the animals received irradiation while their bodies were effectively protected. However, even under such conditions, lethality of a dose over 900 rads was 50%.
Apparatus.
Water maze. The apparatus used in all phases of the experiment was a uniformly blue circular pool (2 m in diameter and 55 cm high) filled to a depth of 25 cm with water (20°C). Rats could escape by climbing on a clear Plexiglas cylinder 11 cm in diameter (invisible platform), which stood 1 cm below the water surface in the middle of one of the four quadrants of the pool in Exp. 1, 3, and 4, or on a visible platform in Exp. 2. The pool was located in a large room with the windows carefully covered to eliminate all sources of light. In the light condition, the room was indirectly illuminated by several lamps, allowing the rats to see numerous remote landmarks, including some cabinets, a black curtain, a closed window, and a door. In the dark condition, all sources of light were eliminated, but it was impossible to control all auditory cues produced by external sources of noise.
Tracking system.
During each trial, an infrared light-emitting diode (LED) connected to a counterbalanced thin cable was attached to the animal’s body with a rubber band. An infrared-sensitive television camera was mounted over the center of the pool and used for monitoring the LED movement. Every 100 ms, the computerized tracking system monitored and stored the position of the LED-indicated rat. The same system was used in both darkness and light. The television monitor, power supply, and computer were located in an alcove in one corner of the room and covered by a black curtain. Only one experimenter was present in the room.
Behavioral Training.
Rats navigated in light or complete darkness in different experiments. A trial began by taking the animal from the waiting cage and placing it into the water facing the wall of the pool at one of the four arbitrarily designated points that marked north, east, south, and west. The tracking system was started at the time the animal was released. The maximum time the rat was allowed to swim was 60 s. The trial stopped either after this time had expired or after the animal had found the platform and remained on it for more than 1 s. When the rat failed to find the platform it was led to it. Climbing onto the platform was the only way of escaping from the water. The animal was always allowed to rest there for 15 s before it was returned to the waiting cage. The next trial was started approximately 15 s later. After the last trial, the animal was towel-dried, taken into another room, and put into a cage under a heating lamp before being returned to the home cage.
Histological Procedure.
At the end of the experiments, the rats were given a lethal dose of pentobarbital and were perfused intracardially with 200 ml of 0.1 M sodium phosphate buffer followed by 500 ml of 4% paraformaldehyde (pH 7.4). The brains were removed and stored in fixative for 2 weeks. They were sectioned on the freezing microtome at 60 μm. Every sixth serial section was mounted and stained with cresyl violet. Adjacent sections were stained with a Nissl-like silver method, demonstrating neuronal perikarya (14). Brains of the two animals from the irradiated group that performed best and worst in the behavioral tests, and brains of two control rats were embedded into paraffin blocks, serially sectioned, and stained with cresyl violet. The 10-μm thick serial sections were used for cell counts both in the dentate gyrus and in the Ammon’s horn. The area of the granule cell layer was measured in representative sections of the dorsal hippocampus drawn by the neurolucida program. The comparison of drawings from irradiated and control animals revealed the magnitude of the granule cell loss in individual animals and allowed correlation of morphological results with behavioral deficits. Timm staining was applied to examine the extent of the mossy fiber bundle in several irradiated rats. The animals were perfused by sodium sulfate followed by 3% glutaraldehyde. The brains were removed, sectioned on the Vibratome at 50 μm, and incubated in Timm’s developer that contained gum arabic and silver nitrate (15). After 45 min of incubation in the dark, the reaction was stopped, and the silver fixed by 1% solution of thiosulfate. Sections were counterstained by cresyl violet and covered by DePex. The area of the hilar region and the mossy fiber bundle were drawn from the Timm-stained preparations using the neurolucida program.
Data Analysis.
Results are presented as the mean ± SEM. Because latency and path length measures were highly correlated and yielded similar conclusions, only latency is reported. ANOVA with repeated measures followed by Tukey’s test and Scheffe’s comparisons were used. Student’s t tests for independent or paired values were used where appropriate.
RESULTS
Experiment 1: Invisible Platform in Light.
Six naive irradiated and 6 naive control rats were trained in 10 daily sessions to escape to the underwater platform in the center of the southwest quadrant of the pool. The start positions were randomly distributed by the computer, so that all four starting points were used in a block of four trials. Two blocks made one session (eight trials per session).
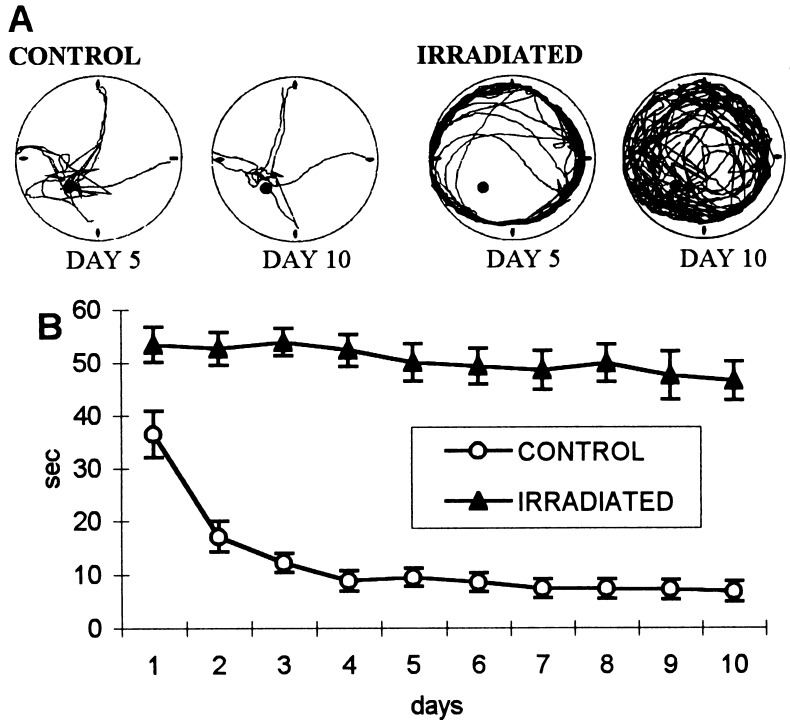
The control group rapidly learned to find the platform and reached on day 4 the asymptotic performance of 8 s, which was not statistically different from the mean 6-s latency in the last 5 days of training (Fig. 1). The irradiated rats swam around the wall of the tank, did not follow the experimenter’s hand leading them to the platform after 60 s, and did not stay on the platform when they found it. Later, most of the irradiated rats learned that an escape platform was somewhere inside the pool and started to leave the wall and search it on a random path (Fig. 1A). They could not navigate, however, averaging an escape latency of 50 s on day 5 and not improving in the last 5 days of training (Fig. 1B). The two groups were significantly different already on day 1 [t(10) = 2.9, P < 0.01].
Figure 1.
(A) Computer printouts of superimposed swimming trajectories of a control and of an irradiated rat, trained in light, to find an invisible platform when started from any of the four cardinal points (north, east, south, west) at the circumference of the pool. Control rats headed toward the target, whereas the irradiated rats swam mostly near the wall of the pool (on day 4). By day 10 the irradiated rats covered the entire surface of the pool. (B) Latency to find the platform in irradiated and control rats. The performance of the control rats improved to the asymptote by day 4, whereas that of the irradiated rats remained almost unchanged, suggesting that irradiated animals are unable to learn the task.
Experiment 2: Visible Platform.
This task, corresponding to simple cue-directed locomotion, can be learned by hippocampectomized rats, but requires intact vision. The same rats and apparatus were used, but the location and form of the platform was changed. The dark gray platform (18 cm in diameter) stood about 1 cm above water surface in the center of the northeast quadrant of the pool. The animals were trained for 3 days.
The control group rapidly learned to escape to the new platform and reached the 5-s asymptote on day 2. Longer escape latency on day 1 [t(5) = 6, P < 0.01] was due to initial search in the previous location of the goal. The irradiated rats had an average latency of 40 s on day 1, and this did not change during the 3 days (Fig. 2). They did not notice the visible platform even when swimming close to it, did not turn, and did not climb upon it.
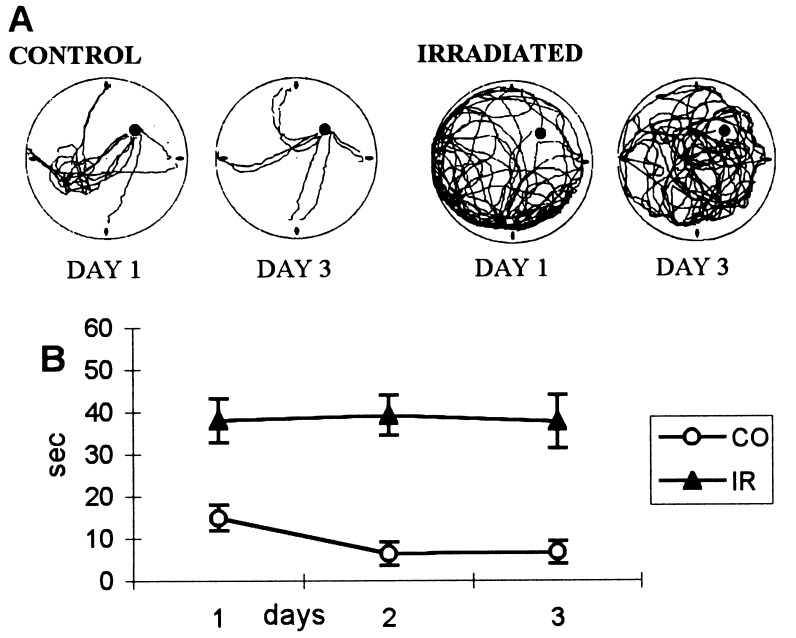
Figure 2.
(A) Computer printouts of the superimposed swimming trajectories of a control and of an irradiated rat, trained in light, to find a visible platform when started from any of the four cardinal points (north, east, south, west) at the perimeter of the pool. The position of the platform was shifted from southwest to northeast. On the first day, the control rats swam initially to southwest, spending a few seconds there before approaching the visible platform in northeast. On day 3, they swam straight to the platform from all starting points. The irradiated rats covered the entire surface of the pool, never achieving a more compact and efficient form. (B) Latency to find the visible platform in control (CO) and irradiated (IR) rats. The control group reached asymptote of 5 s on the second day. The irradiated rats had an average latency of 40 s on day 1, and this did not change during the next two days.
The decrease in average latency from Exp. 1 probably was due to the larger diameter of the platform and increased possibility of chance detection. These results demonstrate that irradiation caused severe visual deficits, interfering with navigational performance.
Experiment 3: Navigation in Complete Darkness.
To decide whether the spatial navigation impairment of the irradiated animals found in Exp. 1 and 2 was caused by granule cell loss in the dentate gyrus or by impaired vision, new groups of naive control and irradiated rats were trained during 4 weeks in complete darkness under conditions of fixed start–fixed target geometry, which were previously shown to be compatible with successful navigation (16).
The procedure described in Materials and Methods was followed. Naive control (n = 8) and irradiated (n = 12) Long–Evans rats were used. The irradiation was performed in a similar way as in Exp. 1 and 2. The windows of the experimental room were carefully covered, and the tracking system, which was located in an alcove in one corner, was separated by a black curtain to eliminate all sources of light and consequently any visual cues.
Rats were trained in 28 consecutive daily sessions. There were typically eight trials per session, except on the first 4 days, when there were four trials per session to reduce the initial stress of the task. The trial began by turning the light off and taking the rat to the starting point in the south on different routes to eliminate a possible visual “fix.” The end of the trial was indicated by turning the lights on either after the rat had found the goal platform (11 cm in diameter) in the center of the northwest quadrant of the pool or after 60 s had elapsed.
The control rats gradually learned to search for the platform in the inner part of the pool. After initial circling and attempts to climb the wall, they started to spend more time in the inner part of the pool and gradually oriented toward the target (Fig. 3A). Their average escape latencies were 40 s in the first week (7 days per week), 24 s in the second, 17 s in the third, and 13 s in the fourth week (Fig. 3B).
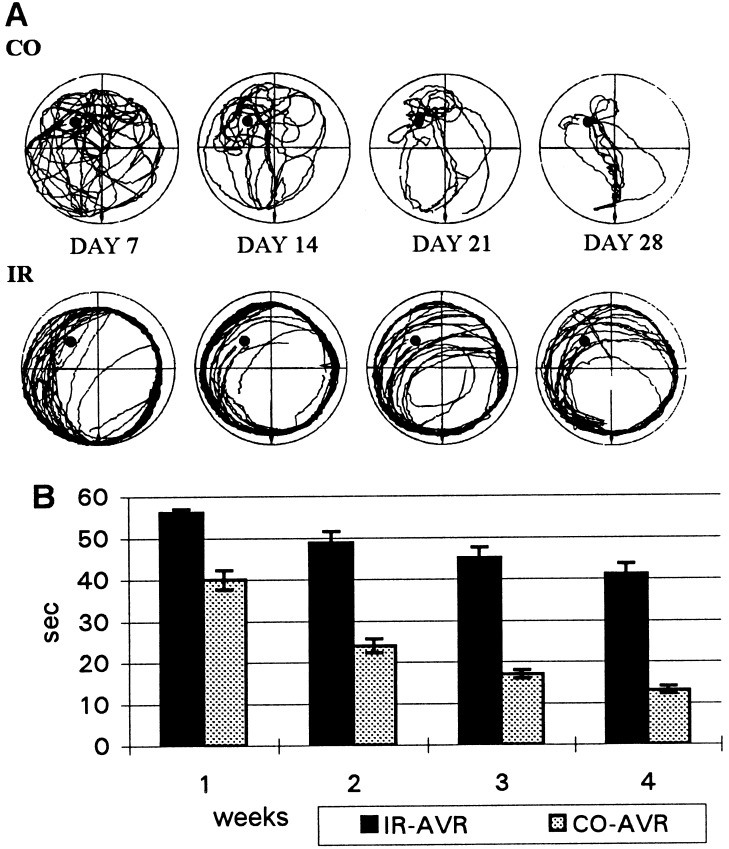
Figure 3.
(A) Computer printouts of superimposed swimming trajectories of control (CO) and irradiated (IR) rats trained in darkness. The rats start at south, and the position of the platform at northwest is indicated by a black dot. The irradiated rats mostly swam close to the wall of the pool and covered a crescent-shaped area around the platform, whereas the trajectories of the control rats contracted gradually to a compact and efficient form by day 28. (B) Latency to find the platform in the dark, under fixed start (south)–fixed goal (northwest) conditions, by the control (CO-AVR) and irradiated (IR-AVR) rats. The columns represent the mean (±SEM) escape latency in 7-day blocks.
The irradiated rats behaved similarly as those in Exp. 1. They swam almost exclusively around the perimeter of the pool (Fig. 3A), and when the light was switched on they did not follow the experimenter’s hand leading them to the platform and did not stay on the platform when placed on it by hand. Later, they learned to rest on the platform, but they still spent most of their 60 s circling around the wall of the pool in one direction. After intensive training, some started to cut arcs from their swimming circles and occasionally found the platform (Fig. 3A). In general, the improvement of the irradiated rats was marginal and slow, but there were substantial differences between individual rats. On some days, the mean escape latency of the best-performing irradiated rat was shorter than the average escape latency of the control group. Nevertheless, the performance of the irradiated group was poor, and its mean escape latency on the fourth week of training did not reach that of the controls on the first week (Fig. 3B). A two-way ANOVA (groups × weeks, 2 × 4) with repeated measures on the last factor revealed significant main effects of groups, F(1, 18) = 85.75, P < 0.0001, and of weeks, F(3, 54) = 91.21, P < 0.0001 as well as significant interaction F(3, 54) = 8.93, P < 0.0001. Scheffe’s comparisons revealed significant differences between the control and irradiated groups within each of the 4 weeks (P < 0.01).
The results of Exp. 3 clearly suggest that the spatial navigation impairment found in Exp. 1 could not only be attributed to the visual deficit, because the irradiated rats also performed significantly worse than intact rats in total darkness.
Experiment 4: Start-Goal Rotation in Darkness.
The purpose of this experiment was to test the relative contributions of allocentric memory and egocentric memory to the poor spatial performance of the irradiated rats. A previous study (16) showed that navigation performance of overtrained control rats in total darkness is at least partly due to allocentric memory for the position of the goal with respect to acoustic beacons. To evaluate the possibility that irradiated rats used allocentric memory, we eliminated the 4 irradiated rats that performed worst in Exp. 3 (group 1) and exposed the remaining 8 irradiated rats (group 2) to a start-goal position change. After a 1-week break, the same method of training was applied in total darkness to 8 rats of group 2 and to 8 controls. After 3 days of retraining, the start and goal positions were rotated by 90° clockwise (from south-northwest to west-northeast). The effect of the start-goal rotation could be observed in the first 5-s trajectories. Although rotation should have no effect on egocentric navigation, the trajectories of the typical control rat shifted in the direction of the allocentric location of the previous goal (Fig. 4A). On the next day, the trajectories of the control rats were somewhat dispersed but were directed more toward the new target position. On the last day of training they assumed a nice compact appearance again (Fig. 4A). On the other hand, the dispersed trajectories of a typical irradiated rat were hardly influenced by the change (Fig. 4A). Even the heading direction of IR01, the best-performing irradiated rat, proved to be quite stable. This suggests that its first 5-s trajectories were generated by egocentric mechanisms (Fig. 4A).
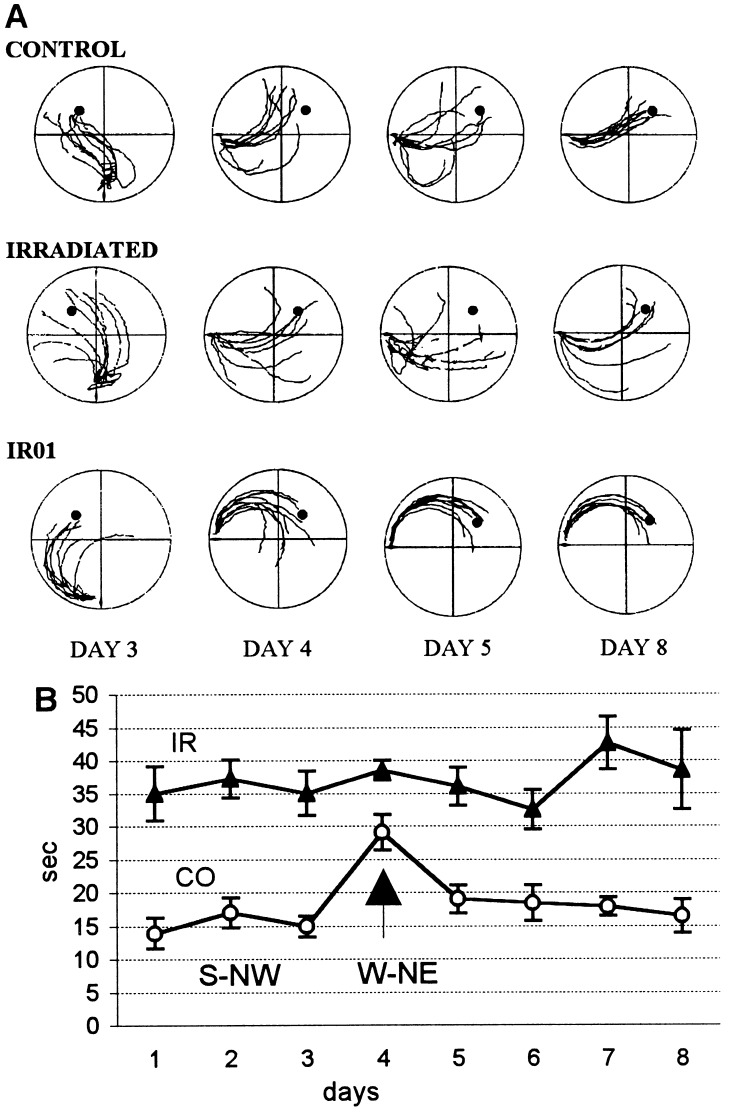
Figure 4.
(A) Computer printouts of the eight superimposed swimming trajectories (first 5 s of each trial) for a typical control rat, a typical irradiated rat, and the best-performing (IR01) irradiated rat. Note that the control rat on the day of change (day 4) swam more toward the previous position of the platform. Their trajectories were somewhat dispersed on day 5, becoming more compact and efficient again by day 8. The dispersed trajectories of the typical irradiated rat were barely affected by the change in platform location on day 4, whereas the heading direction of the best-performing irradiated rat (IR01) was also largely unaffected by the change. (B) Average latency to find the platform on successive days of retraining before and after the platform location was changed to northwest-northeast on day 4 (indicated by arrow). Note that the latency of the control (CO) group almost doubled on day 4, but that no noticeable increase was observed in the irradiated (IR) group.
During the 3 days of retraining the average escape latencies of the control rats were around 15 s, whereas the irradiated animals of group 2 had an average escape latency of 35 s. On the fourth day (the day of change), the mean escape latency of the control animals was almost doubled, whereas that of the irradiated rats remained unchanged (Fig. 4B). The last day of south-northwest training was compared with the first 2 days of west-northeast training using a two-way ANOVA (groups × conditions, 2 × 3) with repeated measures on the last factor that yielded significant main effects of groups [F(1, 14) = 38.70, P < 0.0001] and of conditions [F(2, 28) = 6.52, P < 0.01], as well as significant interaction [F(2, 28) = 3.70, P < 0.05]. Tukey’s protected t tests showed significant difference between the third and the fourth day’s latencies for the control rats (P < 0.01), but not for the irradiated rats.
Light Microscopy of the Irradiated Hippocampus.
In the irradiated animals the number of granule cells and the surface area of the dentate gyrus was greatly reduced (Fig. 5). The surface of granule cell layers decreased to 20–30%, and the hilar region was only one third of that of the controls (Fig. 5 C and D). The number of granule cells in irradiated animals was 150,000-250,000, which corresponds to 25–40% of the control value of 600,000 (17, 18). In the animals that performed worst, the ventral blade of the dentate gyrus was entirely missing, and only a rudiment of the ventral blade was present in the animals who performed better (Fig. 5 B and D). Because the ventral blade of the dentate gyrus contains 50% of the granule cells (17), this result clearly shows the effect of irradiation. In addition, in all irradiated animals, the dorsal blade was greatly reduced (Fig. 5 B and D). Consequently, the mossy fiber bundle (Fig. 5 A and B) that is composed by the axons of granule cells was very thin in the irradiated animals (Fig. 5B). Irradiation happened on the first postnatal day, but between 6 to 18 h after birth, which explains the individual variation in cell loss. The number of pyramidal cells did not change in the CA1–3 areas of Ammon’s horn. In the most severely irradiated animals a shrinkage of the corpus callosum and the parietal cortex was observed.
Figure 5.
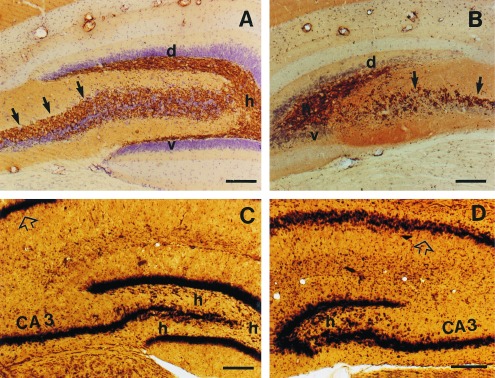
Photomicrographs of the hippocampus in control (A and C) and x-ray irradiated (B and D) rats. In the irradiated rats, the dorsal blade (d) of the dentate gyrus is greatly reduced, whereas the ventral blade (v) is almost completely missing. Furthermore, the hilar region (h) is smaller, and the Timm-labeled mossy fiber bundle (arrows) is sparse in the irradiated animals. The pyramidal cell layer (p) appears not to be affected either in the regio inferior (CA3) or in the regio superior (open arrow) of Ammon’s horn. Cresyl violet counterstained Timm staining (A and B) and Nissl-like silver staining (C and D). Bar = 200 μm.
DISCUSSION
The present study demonstrates that the acquisition of place navigation in the Morris water maze is severely disrupted in adult rats whose brains were exposed shortly after birth to a single high dose of x-ray irradiation. It is conceivable that this deficit can be accounted for by the loss of 60% to 80% of granule cells in the dentate gyrus. Such conclusion must be accepted with caution, however, because irradiation also may damage other brain circuits critical for place navigation.
The results of Exp. 1 indicated that irradiated rats are not able to follow the experimenter’s hand leading them to the goal. The possibility that their vision is severely impaired was confirmed in Exp. 2. The irradiated rats were unable to find a clearly visible escape platform, which was easily located by the control rats. Because such cue-directed locomotion is not disrupted by hippocampectomy (19), the failure of irradiated rats in this task cannot be ascribed to dentate gyrus lesion, but rather to poor vision. Visual loss caused by neonatal irradiation of brain was reported by Stewart-Amidei (20). Histological examination of the retinas of irradiated rats showed significant loss of ganglion cells as compared with controls.
To eliminate the effect of irradiation-induced visual deficit on navigation, new groups of control and irradiated rats used in Exp. 3 learned fixed start–fixed target navigation task in darkness. Acquisition was significantly slower in the irradiated rats than in the control animals. The latter group replicated the results obtained in intact rats (16), which reached an escape latency of 16 s after 4 weeks of training. Although the role of the hippocampus in path integration is not fully understood, the results of Exp. 3 suggest that the failure of irradiated rats to master the fixed start–fixed goal navigation task may be due to the granule cell loss and the ensuing modifications of hippocampal circuitry. The latter factor may account for the differential effect of various types of dentate gyrus lesions on place navigation (3, 8, 9) and place cell activity (9).
The fixed start–fixed goal navigation task in darkness could be used whenever navigation performance might be compromised by possible visual deficits, e.g. in the case of global forebrain ischemia (21). It must be pointed out, however, that this task does not completely eliminate allocentric orientation. Intact rats can use not only egocentric route memory but also allocentric memory for nonvisual extra-maze cues, e.g. acoustic beacons (16). The relatively short escape latency in the control group of Exp. 3 is due to the combined effect of egocentric and allocentric orientation; the relative contributions of these components to the performance of control and irradiated rats was assessed in Exp. 4.
After simultaneous rotation of the start and goal positions, egocentric solution of the task remains correct, whereas allocentric orientation guides the animal incorrectly. The conflict between these two components caused the almost two-fold increase of escape latencies in the control group but no comparable change was seen in the irradiated rats. The trajectories produced during the first 5 s of each trial suggest that the irradiated rats solved the task using egocentric navigation only, which accounts for the slower acquisition rate. Purely egocentric navigation in the fixed target–fixed goal situation has been studied by Save and Moghaddam (22) by preventing allocentric orientation with daily changes of start and goal positions. After 24 days of training under these conditions the mean escape latency was 27 s, i.e. almost twice as long as seen in the present control rats, who were using allocentrically supported egocentric navigation. The performance of irradiated rats is worse, however, than would correspond to the absence of allocentric orientation. Therefore it seems likely, that irradiation also has interfered with the neural circuits supporting egocentric orientation, perhaps including cortical regions participating in the processing of vestibular and kinesthetic signals implementing path integration.
Histological examination showed that the irradiated rats have in addition to the loss of dentate granule cells a thinner corpus callosum and thinner cortex above the hippocampus. The latter change was due to the loss of pyramidal cells in layers III and IV and to a reduced number of glia cells in all cortical layers. The region affected corresponds to the associative parietal cortex; the bilateral lesions in this area impair performance in various spatial tasks requiring allocentric coding of space (23–26) and even in a task based on purely egocentric coding of space (22). On the other hand, the thinner cortex above hippocampus is not comparable with complete bilateral removal of associative posterior cortex. Cortical tissue overlying the hippocampus is routinely damaged in behavioral studies using aspiration lesions of the hippocampus. Sham-operated controls in such studies show that similar cortical lesions do not significantly deteriorate spatial learning (6).
In conclusion, an intact dentate gyrus is critical for hippocampal function in space navigation. Prevention of granule cell formation by neonatal x-ray irradiation is as effective in disrupting navigation as is removal of those cells in adulthood, suggesting that plastic changes of the hippocampal circuits cannot compensate for the loss of granule cells.
Acknowledgments
We thank Al Kaszmiak for critical reading of the manuscript, Gabor Czeh and Tamas Freund for stimulating discussion and Yu. Kaminsky for computer programming. This work was supported by OTKA (Orszagos Tudomanyos Kutatasi Alap; Hungarian Science Foundation) (Grant 017776), the Granting Agency of the Czech Academy of Sciences (Grant 711401), and the McDonnell Foundation (Grant JSMF 95–14).
References
- 1.Nadel L. Hippocampus. 1991;1:221–229. doi: 10.1002/hipo.450010302. [DOI] [PubMed] [Google Scholar]
- 2.O’Keefe J, Nadel L. The Hippocampus as a Cognitive Map. Oxford: Clarendon; 1978. [Google Scholar]
- 3.Sutherland R J, Whishaw I Q, Kolb B. Behav Brain Res. 1983;7:133–153. doi: 10.1016/0166-4328(83)90188-2. [DOI] [PubMed] [Google Scholar]
- 4.O’Keefe J, Dostrovsky J. Brain Res. 1971;34:171–175. doi: 10.1016/0006-8993(71)90358-1. [DOI] [PubMed] [Google Scholar]
- 5.Muller R U, Kubie J L, Ranck J B., Jr J Neurosci. 1987;13:1935–1950. doi: 10.1523/JNEUROSCI.07-07-01935.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moser E, Moser M, Andersen P. J Neurosci. 1993;13:3916–3925. doi: 10.1523/JNEUROSCI.13-09-03916.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stubley-Weartherly L, Harding J W, Wright J W. Brain Res. 1996;716:29–38. doi: 10.1016/0006-8993(95)01589-2. [DOI] [PubMed] [Google Scholar]
- 8.Conrad C D, Roy D J. J Neurosci. 1993;13:2582–2590. doi: 10.1523/JNEUROSCI.13-06-02582.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McNaughton B L, Barnes C A, Meltzer J, Sutherland R J. Exp Brain Res. 1989;76:485–496. doi: 10.1007/BF00248904. [DOI] [PubMed] [Google Scholar]
- 10.Altman J, Anderson W J, Wright K A. Exp Neurol. 1969;24:196–216. doi: 10.1016/0014-4886(69)90015-6. [DOI] [PubMed] [Google Scholar]
- 11.Bayer S A, Altman J. J Comp Neurol. 1975;63:1–19. doi: 10.1002/cne.901630102. [DOI] [PubMed] [Google Scholar]
- 12.Bayer S A, Brunner R L, Hine R, Altman J. Nat New Biol. 1973;242:222–224. doi: 10.1038/newbio242222a0. [DOI] [PubMed] [Google Scholar]
- 13.Mickley G A, Ferguson J L, Nemeth T J, Mulvihill M A, Alderks C E. Behav Neurosci. 1989;103:722–730. doi: 10.1037//0735-7044.103.4.722. [DOI] [PubMed] [Google Scholar]
- 14.Gallyas F, Hsu M, Buzsaki G. J Neurosci Methods. 1993;50:159–164. doi: 10.1016/0165-0270(93)90004-b. [DOI] [PubMed] [Google Scholar]
- 15.Seress L, Ribak C E. J Comp Neurol. 1995;355:93–110. doi: 10.1002/cne.903550111. [DOI] [PubMed] [Google Scholar]
- 16.Moghaddam M, Bures J. Behav Brain Res. 1996;78:121–129. doi: 10.1016/0166-4328(95)00240-5. [DOI] [PubMed] [Google Scholar]
- 17.Seress L, Pokorny J. J Anat. 1981;133:181–195. [PMC free article] [PubMed] [Google Scholar]
- 18.Seress L. J Hirnforschung. 1988;29:335–340. [PubMed] [Google Scholar]
- 19.Nadel L, MacDonald L. Behav Neural Biol. 1980;29:405–409. doi: 10.1016/s0163-1047(80)90430-6. [DOI] [PubMed] [Google Scholar]
- 20.Stewart-Amidei C. Crit Care Nurs Clin North Am. 1995;7:125–133. [PubMed] [Google Scholar]
- 21.Gallyas F, Hsu M, Buzsaki G. Neurosci Lett. 1992;144:177–179. doi: 10.1016/0304-3940(92)90744-r. [DOI] [PubMed] [Google Scholar]
- 22.Save E, Moghaddam M. Behav Neurosci. 1996;110:74–85. doi: 10.1037//0735-7044.110.1.74. [DOI] [PubMed] [Google Scholar]
- 23.Kolb B, Sutherland R J, Whishaw I Q. Behav Neurosci. 1983;97:13–27. doi: 10.1037//0735-7044.97.1.13. [DOI] [PubMed] [Google Scholar]
- 24.Kolb B, Walkey J. Behav Brain Res. 1987;23:127–145. doi: 10.1016/0166-4328(87)90050-7. [DOI] [PubMed] [Google Scholar]
- 25.DiMattia B D, Kesner R P. Behav Neurosci. 1988;102:471–480. doi: 10.1037//0735-7044.102.4.471. [DOI] [PubMed] [Google Scholar]
- 26.Save E, Buhot M C, Foreman N, Thinus-Blanc C. Behav Brain Res. 1992;47:113–127. doi: 10.1016/s0166-4328(05)80118-4. [DOI] [PubMed] [Google Scholar]