Abstract
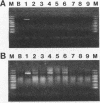
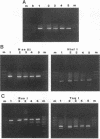
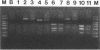
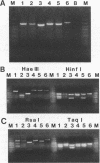
A nonradioactive method to detect Phanerochaete chrysosporium grown in a soil matrix was developed. This method involved DNA extraction, PCR amplification, and restriction enzyme analysis. Amplification of ligninase H8 DNA from pure cultures of P. chrysosporium was not as sensitive as amplification of the internal transcribed spacer (ITS) of the highly repetitive nuclear ribosomal DNA. Amplified ITS DNA was digested with restriction enzymes for analysis. The restriction enzyme pattern of PCR-amplified ITS DNA of P. chrysosporium was unique compared with those of unrelated fungi. Two strains of Phanerochaete chrysosporium and two strains of Phanerochaete sordida were indistinguishable by restriction enzyme analysis, while a third strain of P. chrysosporium had an unique pattern. These results were confirmed by sequence information and indicate that species designations of Phanerochaete spp. should be reexamined. The restriction enzyme pattern of DNA extracted and PCR amplified from P. chrysosporium grown in soil was identical to that from P. chrysosporium grown in pure culture. The ITS sequence was detected in 14 ng of the 100 micrograms of total DNA extracted from 1 g of soil.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alic M., Letzring C., Gold M. H. Mating System and Basidiospore Formation in the Lignin-Degrading Basidiomycete Phanerochaete chrysosporium. Appl Environ Microbiol. 1987 Jul;53(7):1464–1469. doi: 10.1128/aem.53.7.1464-1469.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bumpus J. A., Tien M., Wright D., Aust S. D. Oxidation of persistent environmental pollutants by a white rot fungus. Science. 1985 Jun 21;228(4706):1434–1436. doi: 10.1126/science.3925550. [DOI] [PubMed] [Google Scholar]
- Dietrich D. M., Lamar R. T. Selective Medium for Isolating Phanerochaete chrysosporium from Soil. Appl Environ Microbiol. 1990 Oct;56(10):3088–3092. doi: 10.1128/aem.56.10.3088-3092.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaskell J., Vanden Wymelenberg A., Stewart P., Cullen D. Method to identify specific alleles of a Phanerochaete chrysosporium gene encoding lignin peroxidase. Appl Environ Microbiol. 1992 Apr;58(4):1379–1381. doi: 10.1128/aem.58.4.1379-1381.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picard C., Ponsonnet C., Paget E., Nesme X., Simonet P. Detection and enumeration of bacteria in soil by direct DNA extraction and polymerase chain reaction. Appl Environ Microbiol. 1992 Sep;58(9):2717–2722. doi: 10.1128/aem.58.9.2717-2722.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell P. J., Wagner S., Rodland K. D., Feinbaum R. L., Russell J. P., Bret-Harte M. S., Free S. J., Metzenberg R. L. Organization of the ribosomal ribonucleic acid genes in various wild-type strains and wild-collected strains of Neurospora. Mol Gen Genet. 1984;196(2):275–282. doi: 10.1007/BF00328060. [DOI] [PubMed] [Google Scholar]
- Smith T. L., Schalch H., Gaskell J., Covert S., Cullen D. Nucleotide sequence of a ligninase gene from Phanerochaete chrysosporium. Nucleic Acids Res. 1988 Feb 11;16(3):1219–1219. doi: 10.1093/nar/16.3.1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffan R. J., Atlas R. M. DNA amplification to enhance detection of genetically engineered bacteria in environmental samples. Appl Environ Microbiol. 1988 Sep;54(9):2185–2191. doi: 10.1128/aem.54.9.2185-2191.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tebbe C. C., Vahjen W. Interference of humic acids and DNA extracted directly from soil in detection and transformation of recombinant DNA from bacteria and a yeast. Appl Environ Microbiol. 1993 Aug;59(8):2657–2665. doi: 10.1128/aem.59.8.2657-2665.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai Y. L., Olson B. H. Rapid method for separation of bacterial DNA from humic substances in sediments for polymerase chain reaction. Appl Environ Microbiol. 1992 Jul;58(7):2292–2295. doi: 10.1128/aem.58.7.2292-2295.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wipat A., Wellington E. M., Saunders V. A. Streptomyces marker plasmids for monitoring survival and spread of streptomycetes in soil. Appl Environ Microbiol. 1991 Nov;57(11):3322–3330. doi: 10.1128/aem.57.11.3322-3330.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]