Abstract
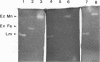
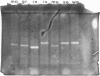
The nature and expression of superoxide dismutase (SOD; EC 1.15.1.1) in the gram-positive food-borne pathogen Listeria monocytogenes were examined. Metal depletion and reconstitution studies and resistance to H2O2 and potassium cyanide inactivation indicated that L. monocytogenes has a single SOD which utilizes manganese as a metal cofactor. The specific activity of SOD was unchanged in cells exposed to a heat shock at 42 degrees C or grown in the presence of paraquat-generated superoxide anion or of metal chelators in the medium. SOD levels increased, however, as the cells progressed through the logarithmic phase of growth and into the stationary phase. Furthermore, SOD activity decreased with decreasing growth temperatures and declined concurrently with decreased growth when higher concentrations of sodium chloride were added to the medium. Cells grown anaerobically possessed relatively high levels of SOD, although these levels were about 10 to 30% lower than those of aerobically grown bacteria. Different isolates of L. monocytogenes were found to produce approximately equivalent levels of SOD, although greater differences in SOD expression were seen among other species of Listeria. When compared with L. monocytogenes, for example, Listeria welshimeri typically produced about 30% greater SOD activity, whereas Listeria murrayi produced about 60% less total SOD activity. Although all species of Listeria produced a single Mn-type SOD, differences in the relative electrophoretic mobility of the native enzymes were noted. These data suggest that the single L. monocytogenes SOD enzyme is constitutively produced in response to many environmental factors and may also be responsive to the cellular growth rate.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersen P., Askgaard D., Ljungqvist L., Bennedsen J., Heron I. Proteins released from Mycobacterium tuberculosis during growth. Infect Immun. 1991 Jun;59(6):1905–1910. doi: 10.1128/iai.59.6.1905-1910.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaman L., Beaman B. L. Monoclonal antibodies demonstrate that superoxide dismutase contributes to protection of Nocardia asteroides within the intact host. Infect Immun. 1990 Sep;58(9):3122–3128. doi: 10.1128/iai.58.9.3122-3128.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchamp C., Fridovich I. Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem. 1971 Nov;44(1):276–287. doi: 10.1016/0003-2697(71)90370-8. [DOI] [PubMed] [Google Scholar]
- Brehm K., Haas A., Goebel W., Kreft J. A gene encoding a superoxide dismutase of the facultative intracellular bacterium Listeria monocytogenes. Gene. 1992 Sep 1;118(1):121–125. doi: 10.1016/0378-1119(92)90258-q. [DOI] [PubMed] [Google Scholar]
- Clare D. A., Duong M. N., Darr D., Archibald F., Fridovich I. Effects of molecular oxygen on detection of superoxide radical with nitroblue tetrazolium and on activity stains for catalase. Anal Biochem. 1984 Aug 1;140(2):532–537. doi: 10.1016/0003-2697(84)90204-5. [DOI] [PubMed] [Google Scholar]
- Dallmier A. W., Martin S. E. Catalase and superoxide dismutase activities after heat injury of Listeria monocytogenes. Appl Environ Microbiol. 1988 Feb;54(2):581–582. doi: 10.1128/aem.54.2.581-582.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallmier A. W., Martin S. E. Catalase, superoxide dismutase, and hemolysin activities and heat susceptibility of Listeria monocytogenes after growth in media containing sodium chloride. Appl Environ Microbiol. 1990 Sep;56(9):2807–2810. doi: 10.1128/aem.56.9.2807-2810.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deneer H. G., Boychuk I. Reduction of ferric iron by Listeria monocytogenes and other species of Listeria. Can J Microbiol. 1993 May;39(5):480–485. doi: 10.1139/m93-068. [DOI] [PubMed] [Google Scholar]
- Deneer H. G., Boychuk I. Species-specific detection of Listeria monocytogenes by DNA amplification. Appl Environ Microbiol. 1991 Feb;57(2):606–609. doi: 10.1128/aem.57.2.606-609.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farber J. M., Peterkin P. I. Listeria monocytogenes, a food-borne pathogen. Microbiol Rev. 1991 Sep;55(3):476–511. doi: 10.1128/mr.55.3.476-511.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GIRARD K. F., SBARRA A. J., BARDAWIL W. A. Serology of Listeria monocytogenes. I. Characteristics of the soluble hemolysin. J Bacteriol. 1963 Feb;85:349–355. doi: 10.1128/jb.85.2.349-355.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldie H. Regulation of transcription of the Escherichia coli phosphoenolpyruvate carboxykinase locus: studies with pck-lacZ operon fusions. J Bacteriol. 1984 Sep;159(3):832–836. doi: 10.1128/jb.159.3.832-836.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas A., Goebel W. Cloning of a superoxide dismutase gene from Listeria ivanovii by functional complementation in Escherichia coli and characterization of the gene product. Mol Gen Genet. 1992 Jan;231(2):313–322. doi: 10.1007/BF00279805. [DOI] [PubMed] [Google Scholar]
- Hassan H. M., Fridovich I. Paraquat and Escherichia coli. Mechanism of production of extracellular superoxide radical. J Biol Chem. 1979 Nov 10;254(21):10846–10852. [PubMed] [Google Scholar]
- Hassan H. M. Microbial superoxide dismutases. Adv Genet. 1989;26:65–97. doi: 10.1016/s0065-2660(08)60223-0. [DOI] [PubMed] [Google Scholar]
- Hopkin K. A., Papazian M. A., Steinman H. M. Functional differences between manganese and iron superoxide dismutases in Escherichia coli K-12. J Biol Chem. 1992 Dec 5;267(34):24253–24258. [PubMed] [Google Scholar]
- Kadurugamuwa J. L., Anwar H., Brown M. R., Shand G. H., Ward K. H. Media for study of growth kinetics and envelope properties of iron-deprived bacteria. J Clin Microbiol. 1987 May;25(5):849–855. doi: 10.1128/jcm.25.5.849-855.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kathariou S., Rocourt J., Hof H., Goebel W. Levels of Listeria monocytogenes hemolysin are not directly proportional to virulence in experimental infections of mice. Infect Immun. 1988 Feb;56(2):534–536. doi: 10.1128/iai.56.2.534-536.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby T., Blum J., Kahane I., Fridovich I. Distinguishing between Mn-containing and Fe-containing superoxide dismutases in crude extracts of cells. Arch Biochem Biophys. 1980 May;201(2):551–555. doi: 10.1016/0003-9861(80)90544-5. [DOI] [PubMed] [Google Scholar]
- Kluge R. M. Listeriosis--problems and therapeutic options. J Antimicrob Chemother. 1990 Jun;25(6):887–890. doi: 10.1093/jac/25.6.887. [DOI] [PubMed] [Google Scholar]
- Knabel S. J., Walker H. W., Hartman P. A., Mendonca A. F. Effects of growth temperature and strictly anaerobic recovery on the survival of Listeria monocytogenes during pasteurization. Appl Environ Microbiol. 1990 Feb;56(2):370–376. doi: 10.1128/aem.56.2.370-376.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Martin M. E., Byers B. R., Olson M. O., Salin M. L., Arceneaux J. E., Tolbert C. A Streptococcus mutans superoxide dismutase that is active with either manganese or iron as a cofactor. J Biol Chem. 1986 Jul 15;261(20):9361–9367. [PubMed] [Google Scholar]
- Myers E. R., Dallmier A. W., Martin S. E. Sodium chloride, potassium chloride, and virulence in Listeria monocytogenes. Appl Environ Microbiol. 1993 Jul;59(7):2082–2086. doi: 10.1128/aem.59.7.2082-2086.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pine L., Weaver R. E., Carlone G. M., Pienta P. A., Rocourt J., Goebel W., Kathariou S., Bibb W. F., Malcolm G. B. Listeria monocytogenes ATCC 35152 and NCTC 7973 contain a nonhemolytic, nonvirulent variant. J Clin Microbiol. 1987 Nov;25(11):2247–2251. doi: 10.1128/jcm.25.11.2247-2251.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portnoy D. A., Chakraborty T., Goebel W., Cossart P. Molecular determinants of Listeria monocytogenes pathogenesis. Infect Immun. 1992 Apr;60(4):1263–1267. doi: 10.1128/iai.60.4.1263-1267.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Privalle C. T., Fridovich I. Induction of superoxide dismutase in Escherichia coli by heat shock. Proc Natl Acad Sci U S A. 1987 May;84(9):2723–2726. doi: 10.1073/pnas.84.9.2723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokolovic Z., Riedel J., Wuenscher M., Goebel W. Surface-associated, PrfA-regulated proteins of Listeria monocytogenes synthesized under stress conditions. Mol Microbiol. 1993 Apr;8(2):219–227. doi: 10.1111/j.1365-2958.1993.tb01566.x. [DOI] [PubMed] [Google Scholar]
- Stephens J. C., Roberts I. S., Jones D., Andrew P. W. Effect of growth temperature on virulence of strains of Listeria monocytogenes in the mouse: evidence for a dose dependence. J Appl Bacteriol. 1991 Mar;70(3):239–244. doi: 10.1111/j.1365-2672.1991.tb02931.x. [DOI] [PubMed] [Google Scholar]
- Welch D. F., Sword C. P., Brehm S., Dusanic D. Relationship between superoxide dismutase and pathogenic mechanisms of Listeria monocytogenes. Infect Immun. 1979 Mar;23(3):863–872. doi: 10.1128/iai.23.3.863-872.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]