Abstract
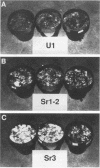
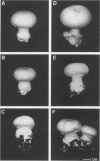
The button mushroom, Agaricus bisporus, is a commercially important cultivated filamentous fungus. During the last decade, the button mushroom industry has depended mainly on two strains (or derivatives of these two strains). Using one of these highly successful strains (strain U1) we examined the phenomenon of strain instability, specifically, the production of irreversible sectors. Three “stromatal” and three “fluffy” sectors were compared with a healthy type U1 strain and with a wild-collected isolate. Compost colonization and fruit body morphology were examined. The main objective of this study, however, was to examine the meiotic stability of the sectored phenotype. Single basidiospores were isolated and subjected to a grain bioassay in which the ability to produce sectors was measured. Our results were as follows: (i) basidiospore cultures obtained from a wild-collected isolate showed no tendency to produce sectors; (ii) approximately 5% of the basidiospore cultures obtained from healthy type U1 strains produced irreversible sectors in the grain bioassay; (iii) the five primary sectors examined produced basidiospore cultures, half of which produced normal-looking growth in the grain bioassay and half of which produced some degree of sectoring; and (iv) the one sectored isolate that represented the F2 generation gave ratios similar to the 1:1 ratio observed for the F1 cultures.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Azevedo J. L., Roper J. A. Mitotic non-conformity in Aspergillus: successive and transposable genetic changes. Genet Res. 1970 Aug;16(1):79–93. doi: 10.1017/s0016672300002299. [DOI] [PubMed] [Google Scholar]
- Castle A. J., Horgen P. A., Anderson J. B. Restriction fragment length polymorphisms in the mushrooms Agaricus brunnescens and Agaricus bitorquis. Appl Environ Microbiol. 1987 Apr;53(4):816–822. doi: 10.1128/aem.53.4.816-822.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DePaola A., Roberts M. C. Class D and E tetracycline resistance determinants in gram-negative bacteria from catfish ponds. Mol Cell Probes. 1995 Oct;9(5):311–313. doi: 10.1016/s0890-8508(95)91572-9. [DOI] [PubMed] [Google Scholar]
- JINKS J. L. Somatic selection in fungi. Nature. 1954 Aug 28;174(4426):409–410. doi: 10.1038/174409b0. [DOI] [PubMed] [Google Scholar]
- Kerrigan R. W., Royer J. C., Baller L. M., Kohli Y., Horgen P. A., Anderson J. B. Meiotic behavior and linkage relationships in the secondarily homothallic fungus Agaricus bisporus. Genetics. 1993 Feb;133(2):225–236. doi: 10.1093/genetics/133.2.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magill J. M., Magill C. W. DNA methylation in fungi. Dev Genet. 1989;10(2):63–69. doi: 10.1002/dvg.1020100202. [DOI] [PubMed] [Google Scholar]
- Mayne R. Y., Bennett J. W., Tallant J. Instability of an aflatoxin-producing strain of Aspergillus parasiticus. Mycologia. 1971 May-Jun;63(3):644–648. [PubMed] [Google Scholar]
- Torres J., Guarro J., Suarez G., Suñe N., Calvo M. A., Ramírez C. Morphological changes in strains of Aspergillus flavus Link ex Fries and Aspergillus parasiticus Speare related with aflatoxin production. Mycopathologia. 1980 Nov 28;72(3):171–180. doi: 10.1007/BF00572660. [DOI] [PubMed] [Google Scholar]