Abstract
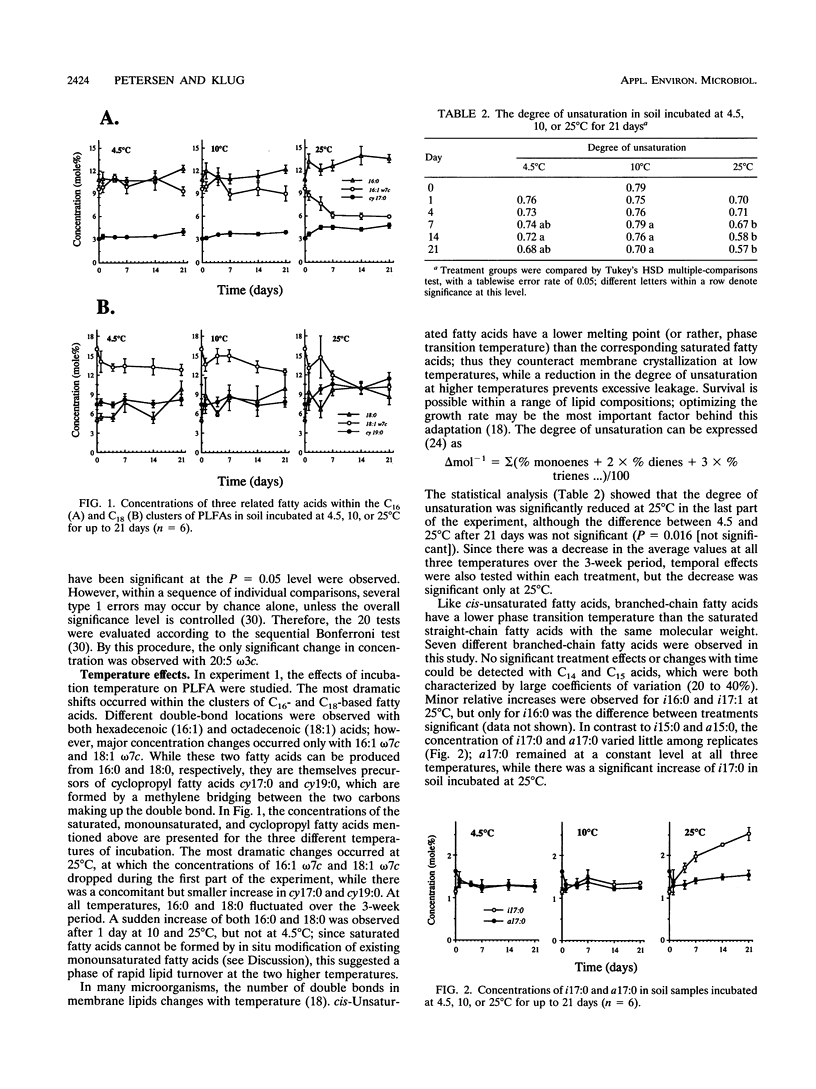
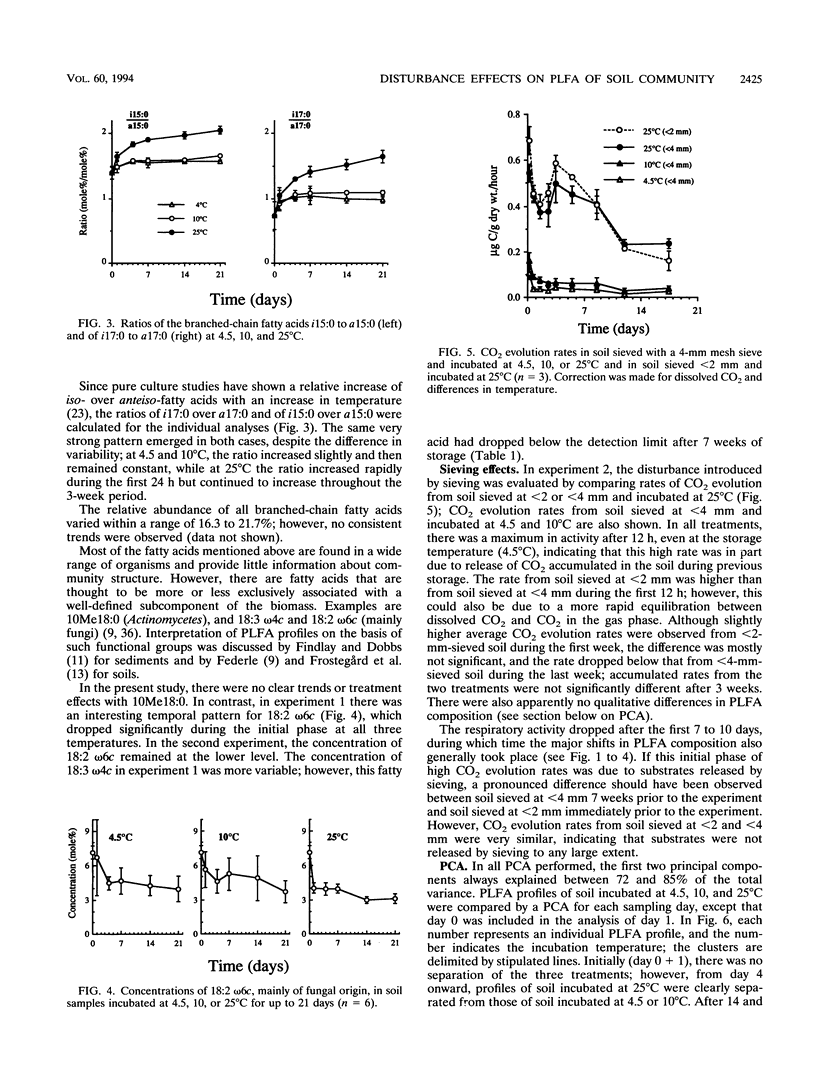
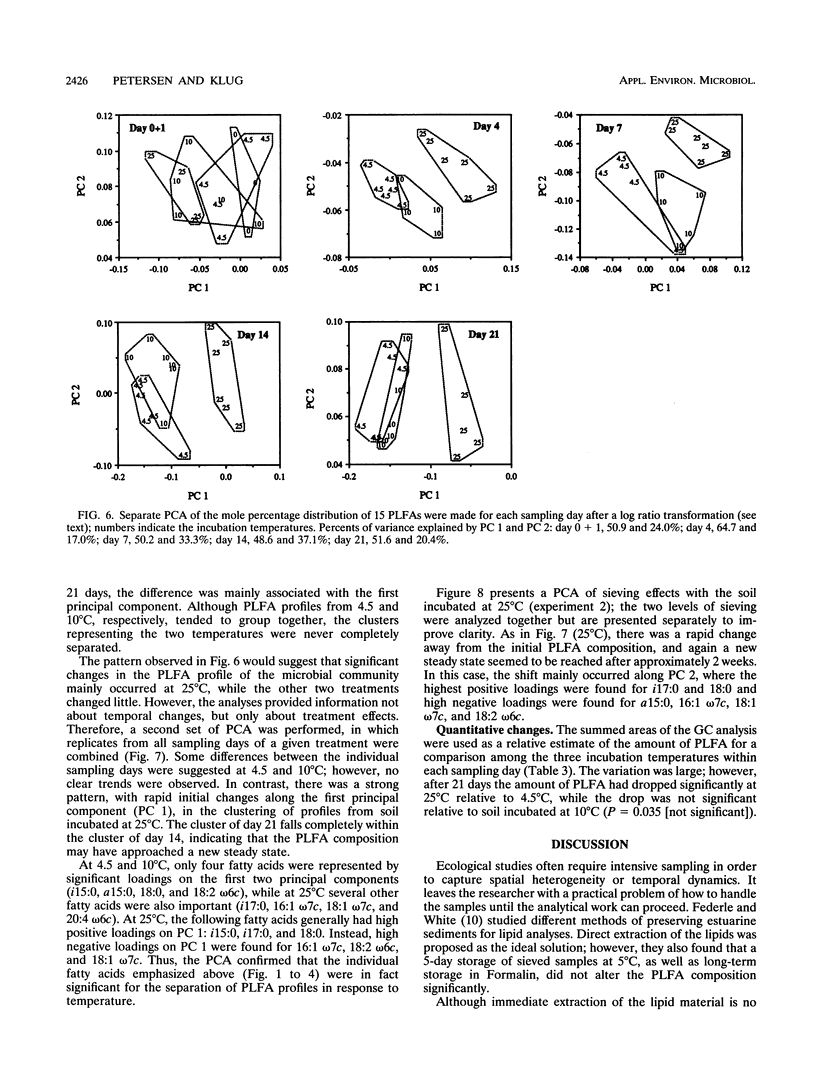
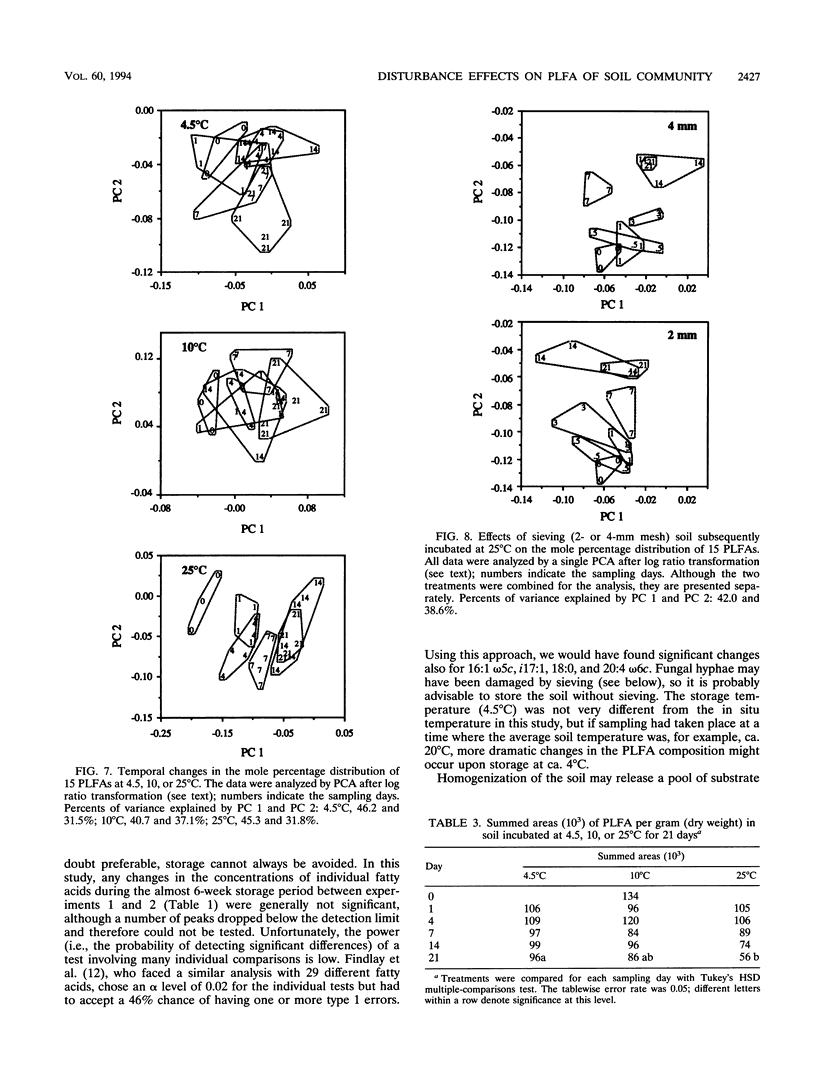
Disturbances typically associated with the study of soil microbial communities, i.e., sieving, storage, and subsequent incubation at elevated temperatures, were investigated with phospholipid fatty acid (PLFA) analyses. Treatment effects were quantified by statistical analyses of the mole percentage distribution of the individual fatty acids. Changes in the concentrations of individual fatty acids over a 7-week storage period at 4.5°C were generally not statistically significant. Sieving effects (mesh size, 4 or 2 mm) on CO2 evolution and the PLFA profile were monitored over 3 weeks; the physical disturbance had only minor effects, although some damage to fungal hyphae by the first sieving (<4 mm) was suggested by a decrease in the signature fatty acid 18:2 ω6c. Temperature effects were investigated by incubating soil for up to 3 weeks at 4.5, 10, or 25°C. Principal component analyses demonstrated a significant shift in the PLFA composition at 25°C over the first 2 weeks, while changes at the other two temperatures were minor. Several of the changes observed at 25°C could be explained with reference to mechanisms of temperature adaptation or as a response to conditions of stress, including a decrease in the degree of unsaturation, an increased production of cyclopropyl fatty acids, and increased ratios of the branched-chain fatty acids iso-15:0 and iso-17:0 over anteiso-15:0 and anteiso-17:0, respectively. A decrease in the total amount of PLFA was also indicated.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson J. P., Domsch K. H. Measurement of bacterial and fungal contributions to respiration of selected agricultural and forest soils. Can J Microbiol. 1975 Mar;21(3):314–322. doi: 10.1139/m75-045. [DOI] [PubMed] [Google Scholar]
- Båth E., Frostegård A., Fritze H. Soil Bacterial Biomass, Activity, Phospholipid Fatty Acid Pattern, and pH Tolerance in an Area Polluted with Alkaline Dust Deposition. Appl Environ Microbiol. 1992 Dec;58(12):4026–4031. doi: 10.1128/aem.58.12.4026-4031.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Federle T. W., White D. C. Preservation of estuarine sediments for lipid analysis of biomass and community structure of microbiota. Appl Environ Microbiol. 1982 Nov;44(5):1166–1169. doi: 10.1128/aem.44.5.1166-1169.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guckert J. B., Hood M. A., White D. C. Phospholipid ester-linked fatty acid profile changes during nutrient deprivation of Vibrio cholerae: increases in the trans/cis ratio and proportions of cyclopropyl fatty acids. Appl Environ Microbiol. 1986 Oct;52(4):794–801. doi: 10.1128/aem.52.4.794-801.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaluzny M. A., Duncan L. A., Merritt M. V., Epps D. E. Rapid separation of lipid classes in high yield and purity using bonded phase columns. J Lipid Res. 1985 Jan;26(1):135–140. [PubMed] [Google Scholar]
- Kaneda T. Iso- and anteiso-fatty acids in bacteria: biosynthesis, function, and taxonomic significance. Microbiol Rev. 1991 Jun;55(2):288–302. doi: 10.1128/mr.55.2.288-302.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas T. D., Batt R. D. Degradation of cell constituents by starved Streptococcus lactis in relation to survival. J Gen Microbiol. 1969 Nov;58(3):347–362. doi: 10.1099/00221287-58-3-347. [DOI] [PubMed] [Google Scholar]



