Abstract
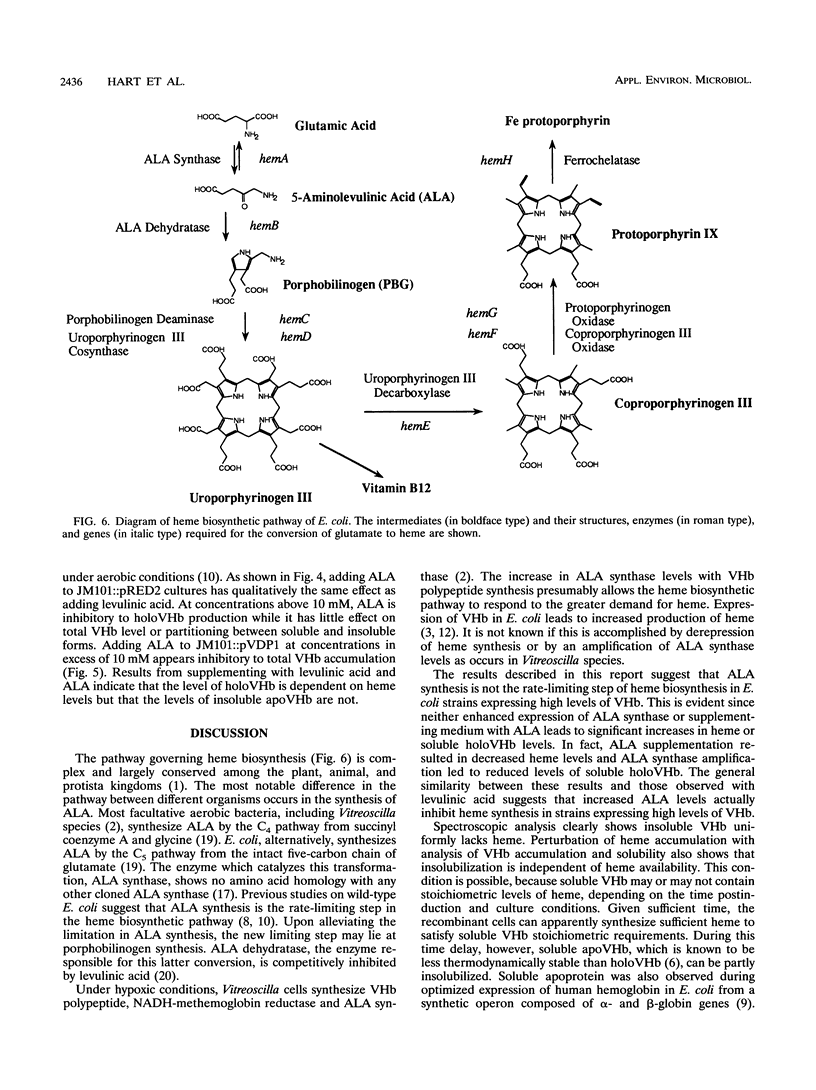
Vitreoscilla hemoglobin (VHb) is accumulated at high levels in both soluble and insoluble forms when expressed from its native promoter on a pUC19-derived plasmid in Escherichia coli. Examination by atomic absorption spectroscopy and electron paramagnetic resonance spectroscopy revealed that the insoluble form uniformly lacks the heme prosthetic group (apoVHb). The purified soluble form contains heme (holoVHb) and is spectroscopically indistinguishable from holoVHb produced by Vitreoscilla cells. This observation suggested that a relationship may exist between the insolubility of apoVHb and biosynthesis of heme. To examine this possibility, a series of experiments were conducted to chemically and genetically manipulate the formation and conversion of 5-aminolevulinic acid (ALA), a key intermediate in heme biosynthesis. Chemical perturbations involved supplementing the growth medium with the intermediate ALA and the competitive inhibitor levulinic acid which freely cross the cell barrier. Genetic manipulations involved amplifying the gene dosage for the enzymes ALA synthase and ALA dehydratase. Results from both levulinic acid and ALA supplementations indicate that the level of soluble holoVHb correlates with the heme level but that the level of insoluble apoVHb does not. The ratio of soluble to insoluble VHb also does not correlate with the level of total VHb accumulated. The effect of amplifying ALA synthase and ALA dehydratase gene dosage is complex and may involve secondary factors. Results indicate that the rate-limiting step of heme biosynthesis in cells overproducing VHb does not lie at ALA synthesis, as it reportedly does in wild-type E. coli (S. Hino and A. Ishida, Enzyme 16:42-49, 1973).
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dikshit K. L., Spaulding D., Braun A., Webster D. A. Oxygen inhibition of globin gene transcription and bacterial haemoglobin synthesis in Vitreoscilla. J Gen Microbiol. 1989 Oct;135(10):2601–2609. doi: 10.1099/00221287-135-10-2601. [DOI] [PubMed] [Google Scholar]
- Dikshit K. L., Webster D. A. Cloning, characterization and expression of the bacterial globin gene from Vitreoscilla in Escherichia coli. Gene. 1988 Oct 30;70(2):377–386. doi: 10.1016/0378-1119(88)90209-0. [DOI] [PubMed] [Google Scholar]
- Hart R. A., Bailey J. E. Purification and aqueous two-phase partitioning properties of recombinant Vitreoscilla hemoglobin. Enzyme Microb Technol. 1991 Oct;13(10):788–795. doi: 10.1016/0141-0229(91)90061-e. [DOI] [PubMed] [Google Scholar]
- Hart R. A., Rinas U., Bailey J. E. Protein composition of Vitreoscilla hemoglobin inclusion bodies produced in Escherichia coli. J Biol Chem. 1990 Jul 25;265(21):12728–12733. [PubMed] [Google Scholar]
- Hino S., Ishida A. Effect of oxygen on heme and cytochrome content in some facultative bacteria. Enzyme. 1973;16(1):42–49. doi: 10.1159/000459360. [DOI] [PubMed] [Google Scholar]
- Hoffman S. J., Looker D. L., Roehrich J. M., Cozart P. E., Durfee S. L., Tedesco J. L., Stetler G. L. Expression of fully functional tetrameric human hemoglobin in Escherichia coli. Proc Natl Acad Sci U S A. 1990 Nov;87(21):8521–8525. doi: 10.1073/pnas.87.21.8521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khosla C., Bailey J. E. Characterization of the oxygen-dependent promoter of the Vitreoscilla hemoglobin gene in Escherichia coli. J Bacteriol. 1989 Nov;171(11):5995–6004. doi: 10.1128/jb.171.11.5995-6004.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khosla C., Bailey J. E. Evidence for partial export of Vitreoscilla hemoglobin into the periplasmic space in Escherichia coli. Implications for protein function. J Mol Biol. 1989 Nov 5;210(1):79–89. doi: 10.1016/0022-2836(89)90292-1. [DOI] [PubMed] [Google Scholar]
- Khosla C., Bailey J. E. Heterologous expression of a bacterial haemoglobin improves the growth properties of recombinant Escherichia coli. Nature. 1988 Feb 18;331(6157):633–635. doi: 10.1038/331633a0. [DOI] [PubMed] [Google Scholar]
- Khosla C., Bailey J. E. The Vitreoscilla hemoglobin gene: molecular cloning, nucleotide sequence and genetic expression in Escherichia coli. Mol Gen Genet. 1988 Sep;214(1):158–161. doi: 10.1007/BF00340195. [DOI] [PubMed] [Google Scholar]
- Li J. M., Brathwaite O., Cosloy S. D., Russell C. S. 5-Aminolevulinic acid synthesis in Escherichia coli. J Bacteriol. 1989 May;171(5):2547–2552. doi: 10.1128/jb.171.5.2547-2552.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J. M., Russell C. S., Cosloy S. D. Cloning and structure of the hem A gene of Escherichia coli K-12. Gene. 1989 Oct 30;82(2):209–217. doi: 10.1016/0378-1119(89)90046-2. [DOI] [PubMed] [Google Scholar]
- Li J. M., Russell C. S., Cosloy S. D. The structure of the Escherichia coli hemB gene. Gene. 1989 Jan 30;75(1):177–184. doi: 10.1016/0378-1119(89)90394-6. [DOI] [PubMed] [Google Scholar]
- Li J. M., Umanoff H., Proenca R., Russell C. S., Cosloy S. D. Cloning of the Escherichia coli K-12 hemB gene. J Bacteriol. 1988 Feb;170(2):1021–1025. doi: 10.1128/jb.170.2.1021-1025.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orii Y., Webster D. A. Photodissociation of oxygenated cytochrome o(s) (Vitreoscilla) and kinetic studies of reassociation. J Biol Chem. 1986 Mar 15;261(8):3544–3547. [PubMed] [Google Scholar]
- Wakabayashi S., Matsubara H., Webster D. A. Primary sequence of a dimeric bacterial haemoglobin from Vitreoscilla. 1986 Jul 31-Aug 6Nature. 322(6078):481–483. doi: 10.1038/322481a0. [DOI] [PubMed] [Google Scholar]
- Wang L. F., Hum W. T., Kalyan N. K., Lee S. G., Hung P. P., Doi R. H. Synthesis and refolding of human tissue-type plasminogen activator in Bacillus subtilis. Gene. 1989 Dec 7;84(1):127–133. doi: 10.1016/0378-1119(89)90146-7. [DOI] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]