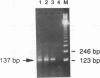
Abstract
The pyruvate kinase gene pyk from Corynebacterium glutamicum was cloned by applying a combination of PCR, site-specific mutagenesis, and complementation. A 126-bp DNA fragment central to the C. glutamicum pyk gene was amplified from genomic DNA by PCR with degenerate oligonucleotides as primers. The cloned DNA fragment was used to inactivate the pyk gene in C. glutamicum by marker rescue mutagenesis via homologous recombination. The C. glutamicum pyk mutant obtained was unable to grow on minimal medium containing ribose as the sole carbon source. Complementation of this phenotype by a gene library resulted in the isolation of a 2.8-kb PstI-BamHI genomic DNA fragment harboring the C. glutamicum pyk gene. Multiple copies of plasmid-borne pyk caused a 20-fold increase of pyruvate kinase activity in C. glutamicum cell extracts. By using large internal fragments of the cloned C. glutamicum gene, pyk mutant derivatives of the lysine production strain Corynebacterium lactofermentum 21799 were generated by marker rescue mutagenesis. As determined in shake flask fermentations, lysine production in pyk mutants was 40% lower than that in the pyk+ parent strain, indicating that pyruvate kinase is essential for high-level lysine production. This finding questions an earlier hypothesis postulating that redirection of carbon flow at the phosphoenol pyruvate branch point of glycolysis through elimination of pyruvate kinase activity results in an increase of lysine production in C. glutamicum and its close relatives.
Full text
PDF


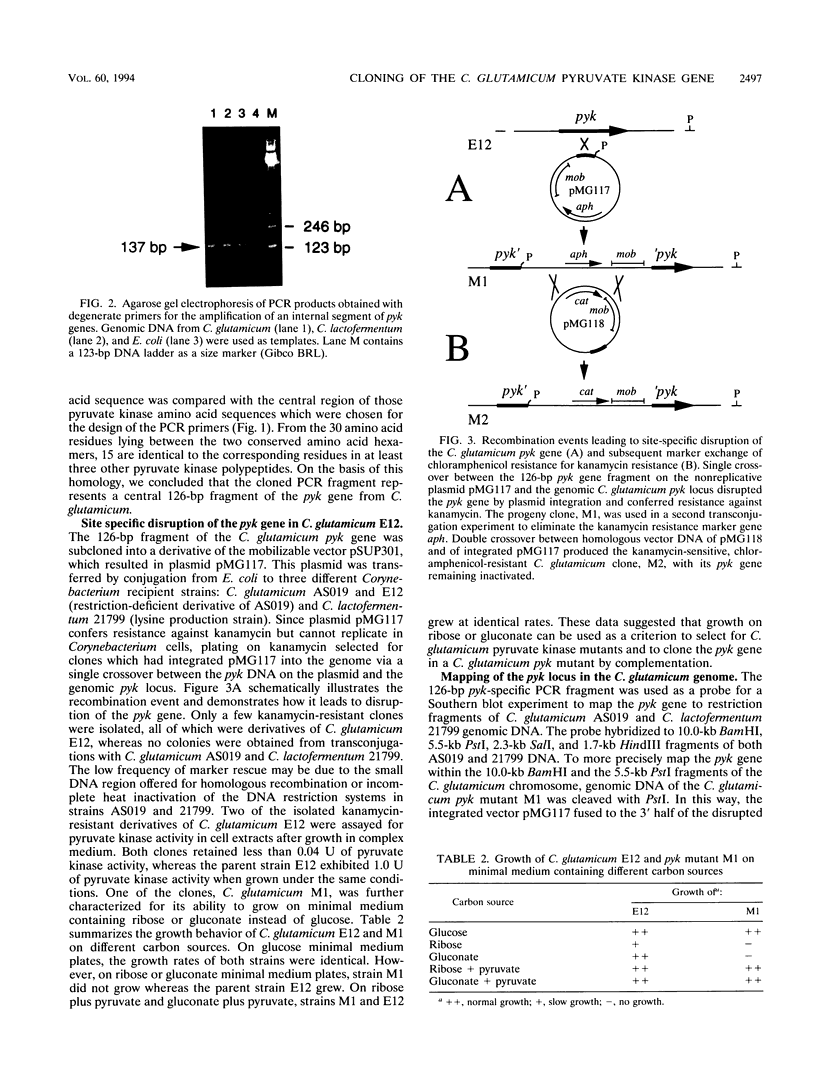
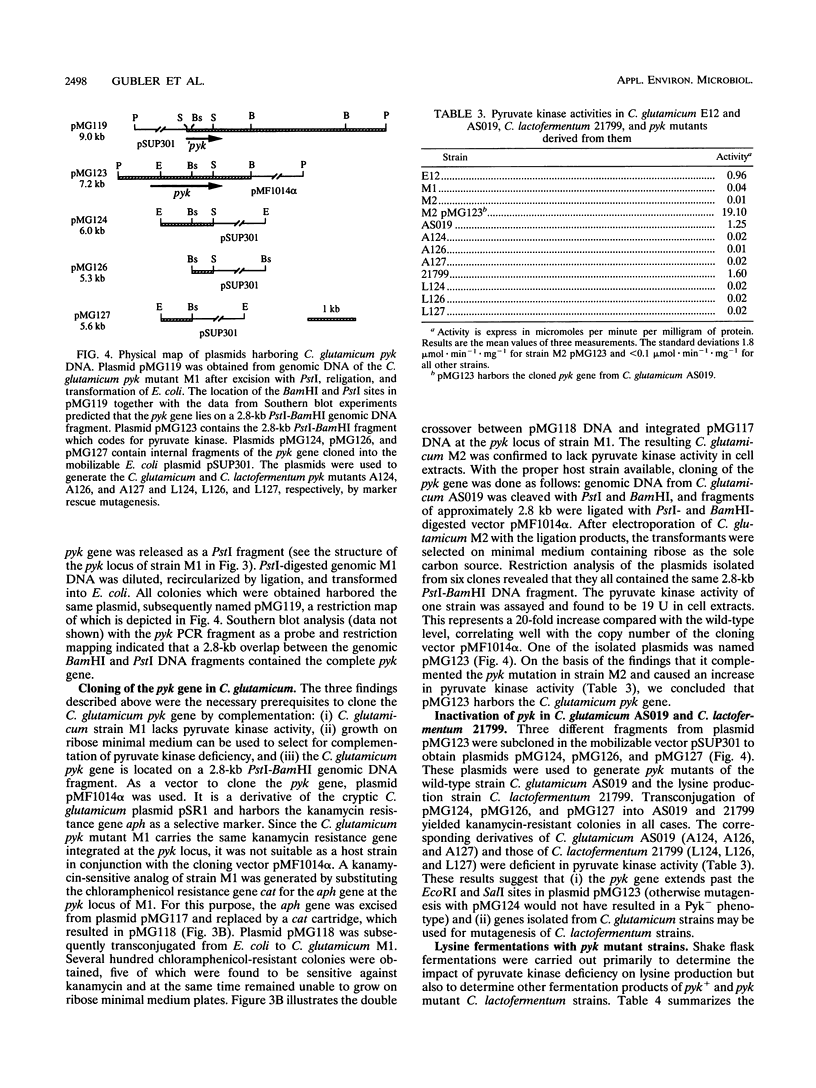


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burke R. L., Tekamp-Olson P., Najarian R. The isolation, characterization, and sequence of the pyruvate kinase gene of Saccharomyces cerevisiae. J Biol Chem. 1983 Feb 25;258(4):2193–2201. [PubMed] [Google Scholar]
- Duwat P., Ehrlich S. D., Gruss A. Use of degenerate primers for polymerase chain reaction cloning and sequencing of the Lactococcus lactis subsp. lactis recA gene. Appl Environ Microbiol. 1992 Aug;58(8):2674–2678. doi: 10.1128/aem.58.8.2674-2678.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Follettie M. T., Peoples O. P., Agoropoulou C., Sinskey A. J. Gene structure and expression of the Corynebacterium flavum N13 ask-asd operon. J Bacteriol. 1993 Jul;175(13):4096–4103. doi: 10.1128/jb.175.13.4096-4103.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983 Jun 5;166(4):557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- Llanos R. M., Harris C. J., Hillier A. J., Davidson B. E. Identification of a novel operon in Lactococcus lactis encoding three enzymes for lactic acid synthesis: phosphofructokinase, pyruvate kinase, and lactate dehydrogenase. J Bacteriol. 1993 May;175(9):2541–2551. doi: 10.1128/jb.175.9.2541-2551.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyajima R., Shiio I. Regulation of aspartate family amino acid biosynthesis in Brevibacterium flavum. 3. Properties of homoserine dehydrogenase. J Biochem. 1970 Sep;68(3):311–319. doi: 10.1093/oxfordjournals.jbchem.a129361. [DOI] [PubMed] [Google Scholar]
- Ozaki H., Shiio I. Regulation of the TCA and glyoxylate cycles in Brevibacterium flavum. II. Regulation of phosphoenolpyruvate carboxylase and pyruvate kinase. J Biochem. 1969 Sep;66(3):297–311. doi: 10.1093/oxfordjournals.jbchem.a129148. [DOI] [PubMed] [Google Scholar]
- Sakai H., Ohta T. Molecular cloning and nucleotide sequence of the gene for pyruvate kinase of Bacillus stearothermophilus and the production of the enzyme in Escherichia coli. Evidence that the genes for phosphofructokinase and pyruvate kinase constitute an operon. Eur J Biochem. 1993 Feb 1;211(3):851–859. doi: 10.1111/j.1432-1033.1993.tb17618.x. [DOI] [PubMed] [Google Scholar]
- Schwarzer A., Pühler A. Manipulation of Corynebacterium glutamicum by gene disruption and replacement. Biotechnology (N Y) 1991 Jan;9(1):84–87. doi: 10.1038/nbt0191-84. [DOI] [PubMed] [Google Scholar]
- Schäfer A., Kalinowski J., Simon R., Seep-Feldhaus A. H., Pühler A. High-frequency conjugal plasmid transfer from gram-negative Escherichia coli to various gram-positive coryneform bacteria. J Bacteriol. 1990 Mar;172(3):1663–1666. doi: 10.1128/jb.172.3.1663-1666.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephanopoulos G., Vallino J. J. Network rigidity and metabolic engineering in metabolite overproduction. Science. 1991 Jun 21;252(5013):1675–1681. doi: 10.1126/science.1904627. [DOI] [PubMed] [Google Scholar]
- Tani K., Yoshida M. C., Satoh H., Mitamura K., Noguchi T., Tanaka T., Fujii H., Miwa S. Human M2-type pyruvate kinase: cDNA cloning, chromosomal assignment and expression in hepatoma. Gene. 1988 Dec 20;73(2):509–516. doi: 10.1016/0378-1119(88)90515-x. [DOI] [PubMed] [Google Scholar]
- Yoshihama M., Higashiro K., Rao E. A., Akedo M., Shanabruch W. G., Follettie M. T., Walker G. C., Sinskey A. J. Cloning vector system for Corynebacterium glutamicum. J Bacteriol. 1985 May;162(2):591–597. doi: 10.1128/jb.162.2.591-597.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Graaff L., Visser J. Structure of the Aspergillus nidulans pyruvate kinase gene. Curr Genet. 1988 Dec;14(6):553–560. doi: 10.1007/BF00434080. [DOI] [PubMed] [Google Scholar]